Solved With Heart & Science
Articles by technical and scientific subject matter experts
최신 과학 혁신, 약물 개발 혁신, 업계 인사이트 등을 확인하실 수 있습니다. 카테고리 별로 블로그를 검색하고 관련 포스트를 확인하세요.
관심 분야를 선택해 주세요.

Blog post
Enabling flexibility and scientific innovation in oral solid dose development
A strategic CDMO partnership enabled a clinical-stage pharmaceutical company to navigate the complexities of early drug development, advancing formulation strategies, adapting to shifting priorities, and ensuring a scalable path to clinical trials.
12 minute read

Blog post
The real challenge of biologics scale-up isn’t capacity—it’s demand
Most biologics never reach the production volumes that justify large-scale stainless-steel capacity. The real challenge isn’t building enough—it’s knowing how much will truly be needed, and when. Here’s why flexibility in scaling is becoming one of biologics manufacturing’s most strategic advantages.
16 minute read

Blog post
How to Achieve Late-Phase Success in OSD Development
Learn how to achieve late-phase success in oral solid dose scale-up with data-driven strategies, risk mitigation, and the right CDMO partnership.
11 minute read

Blog post
Discussing the Future of Biotech
Explore expert insight into what trends are shaping the biotech industry, including innovations, sustainability, AI, and growth in a dynamic landscape.
10 minute read

Blog post
Accelerate drug development by embracing an integrated approach
Pharmaceutical companies face significant challenges with rising R&D costs and extended development timelines. Embracing an integrated CDMO and CRO approach that combines drug substance, drug product, clinical manufacturing, clinical research, and clinical supply chain management into one streamlined service can simplify complexity, enhance efficiency, and reduce risks, ultimately accelerating the journey from lab to market.
12 minute read
Blog post
Expert perspective: How Thermo Fisher Scientific’s packaging and labeling innovations simplify biotech clinical trials
Find expert insight on how innovative clinical packaging and labeling solutions can streamline processes, enhance efficiency, compliance and overall success.
15 minute read

초기 단계 경구제 제형 개발의 5가지 숨은 리스크
초기 단계 경구제 개발에는 장기적인 성공에 영향을 미칠 수 있는 다양한 숨은 리스크가 존재합니다. API의 복잡성부터 생산 규모 확대 가능성, 규제 대응 준비까지, 신중한 계획 수립은 예상치 못한 차질을 줄이고 개발 프로그램을 원활히 진행하는데 도움이 됩니다.
12 minute read

바이오의약품을 위한 세포주 개발: 안정성과 수율 향상
세포주 개발은 바이오의약품 제조에서 매우 중요한 과정으로 공정 효율성, 생산 규모 확장성, 제품 안정성에 큰 영향을 미칩니다. 최근 CHO K1 세포주 엔지니어링의 발전으로 인해 수율이 향상되고 유전자 안정성이 개선되며 IND 제출까지의 기간도 단축되고 있습니다.
15 minute read

Understanding large molecule drugs
이 블로그에서는 바이오 의약품에 대해 심층적으로 살펴보며, 그 주요 특성, 장점과 과제, 그리고 제약 산업에서의 미래를 다룹니다.
18 minute read

Tech transfer, part 1: 의약품 생산 불확실성 극복하기 - 기술 이전의 핵심 역할
효율적인 기술 이전은 제품 품질 유지, 지적 재산 보호, 비용 관리, 및 생산 규모 확대를 지원하여, 제약 기업이 새로운 기회와 도전에 효과적으로 대응하고 경쟁력을 유지하며, 환자에게 지속적인 의약품 공급을 보장할 수 있도록 합니다.
15 minute read

Blog post
Regulatory landscape in Europe: Key advice for meeting post-Brexit Qualified Person requirements
The United Kingdom’s (UK) departure from the European Union (EU) has added a layer of complexity to the clinical trial supply chain in Europe above and beyond COVID-related disruptions.
(4 minute read)

Blog post
생체시료 저장소 구축의 장단점 비교
생체시료 저장소는 생물학적 물질을 수집, 보존, 활용하는 데 중요한 역할을 합니다. 이를 사내에 구축하는 것과 구입하는 것 중 어떤 것이 합리적일까요?
8 minute read

Blog post
바이러스 벡터 상용화 – Part 2: 프로세스 밸리데이션 주기 모범 사례
바이러스 벡터의 안전성, 유효성 및 품질을 보장하기 위한 다양한 평가 및 시험을 포함하는 바이러스 벡터 공정 밸리데이션 주기에 대해 자세히 알아보세요.
11 minute read

Blog post
CDMO 파트너를 선택할 시 고려해야 할 7가지
CDMO가 제약사와 협력하는 방법에 대해 알아보고 기업이 CDMO 파트너를 선정할 때 고려해야 할 주요 고려사항에 대해 확인하세요.
9 minute read
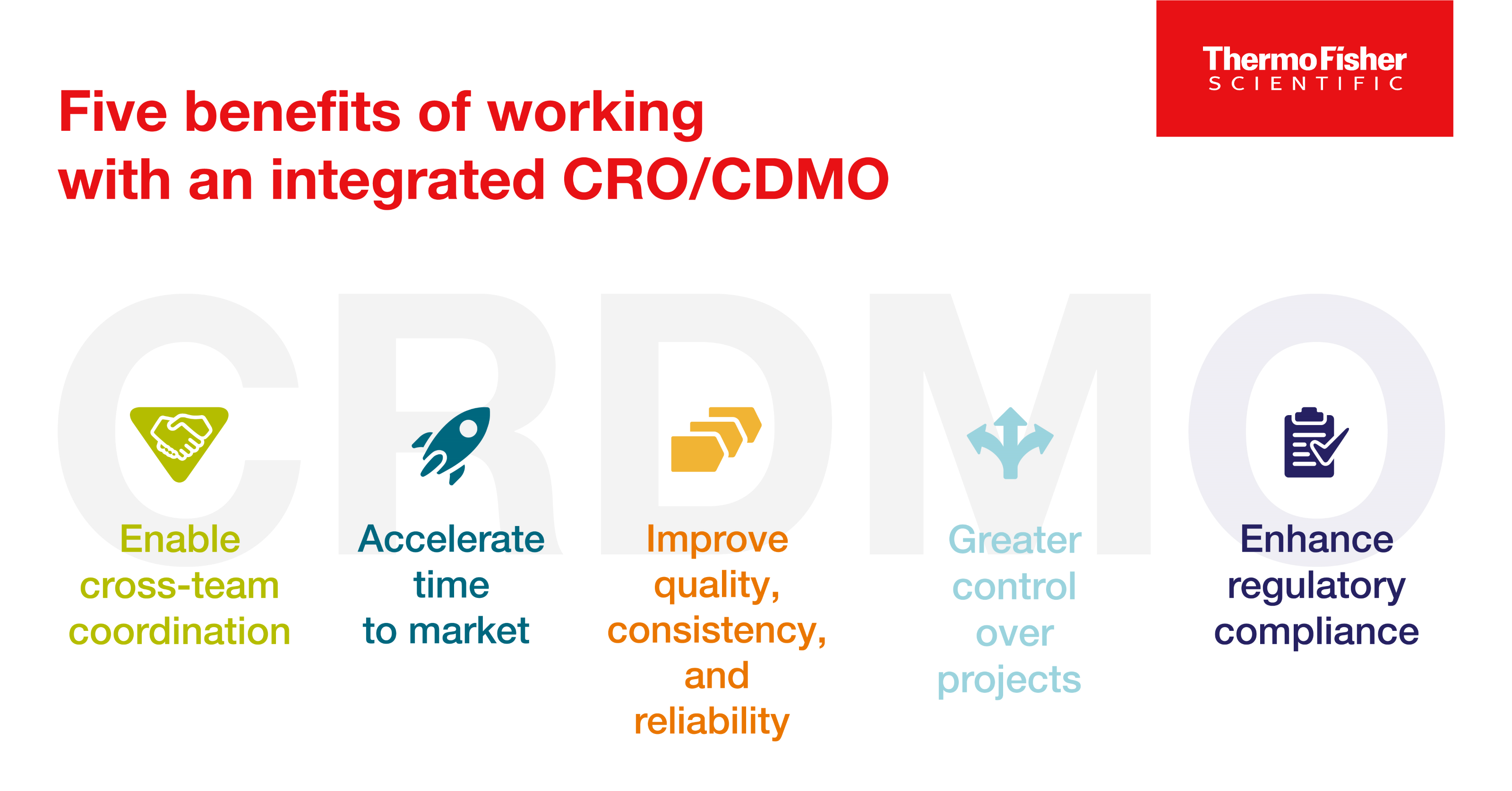
Blog post
CRDMO - CRO/CDMO 통합을 통한 의약품 개발의 재구성
의약품 개발사는 통합 CRO/CDMO와 협력함으로써 여러 아웃소싱 파트너와 협력해야 하는 복잡함 없이 새로운 치료법을 시장에 출시하는 과정을 보다 효율적으로 탐색할 수 있습니다. 통합 CRO/CDMO와 협력할 때 얻을 수 있는 이점에 대해 알아보세요.
9 minute read
Blog post
순응도 및 정확성: 스마트 패키징으로 임상시험에서 품질 필수 요건 개선하기
Thermo Fisher Scientific의 약물 순응도 및 바이오마커 책임자로부터 스마트 패키징의 데이터 품질 영향과 이를 임상시험에 통합하는 방법에 대해 확인하세요.
15 minute read
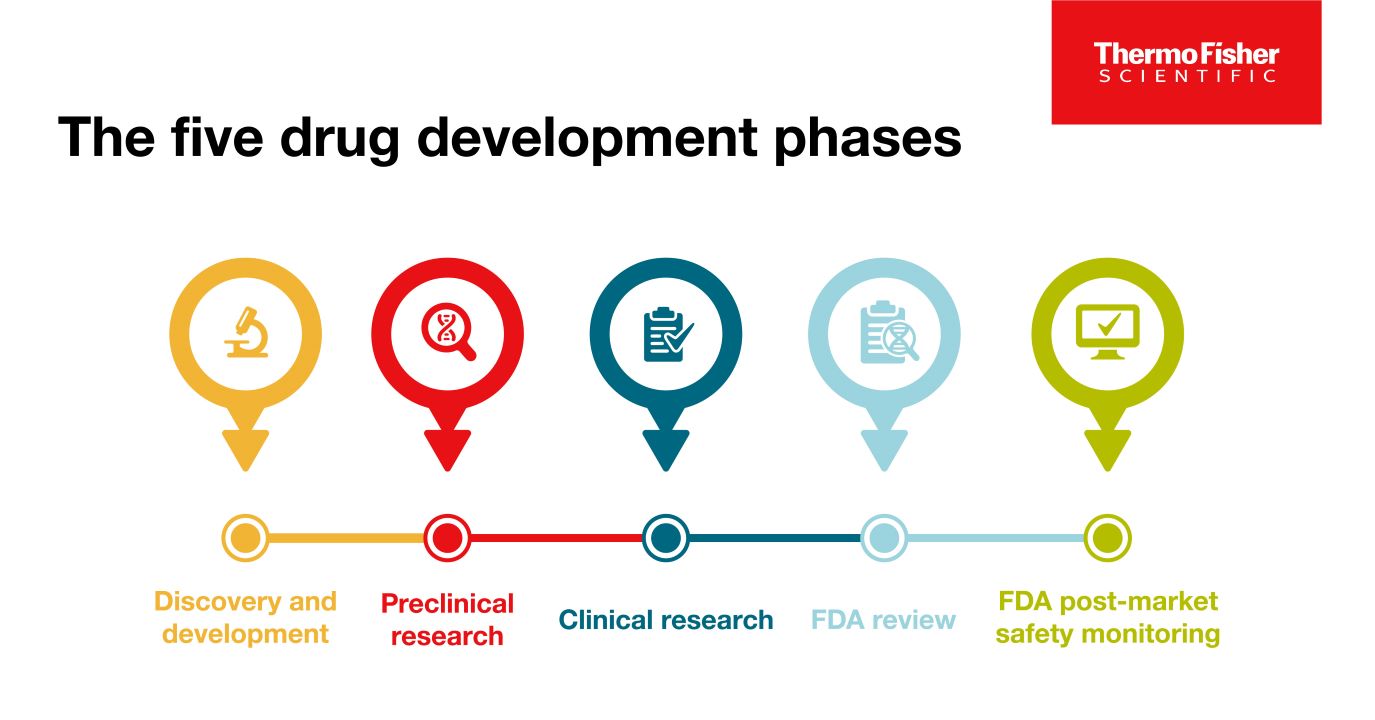
Blog post
약물 개발의 5단계
성공적인 상업화를 위해서, 모든 약물은 1) 약물 발견 및 개발 2) 전임상 연구 3) 임상 연구 4) FDA 검토 5) 안전성 모니터링의 5가지 특정 단계를 통과해야 합니다.
9 minute read
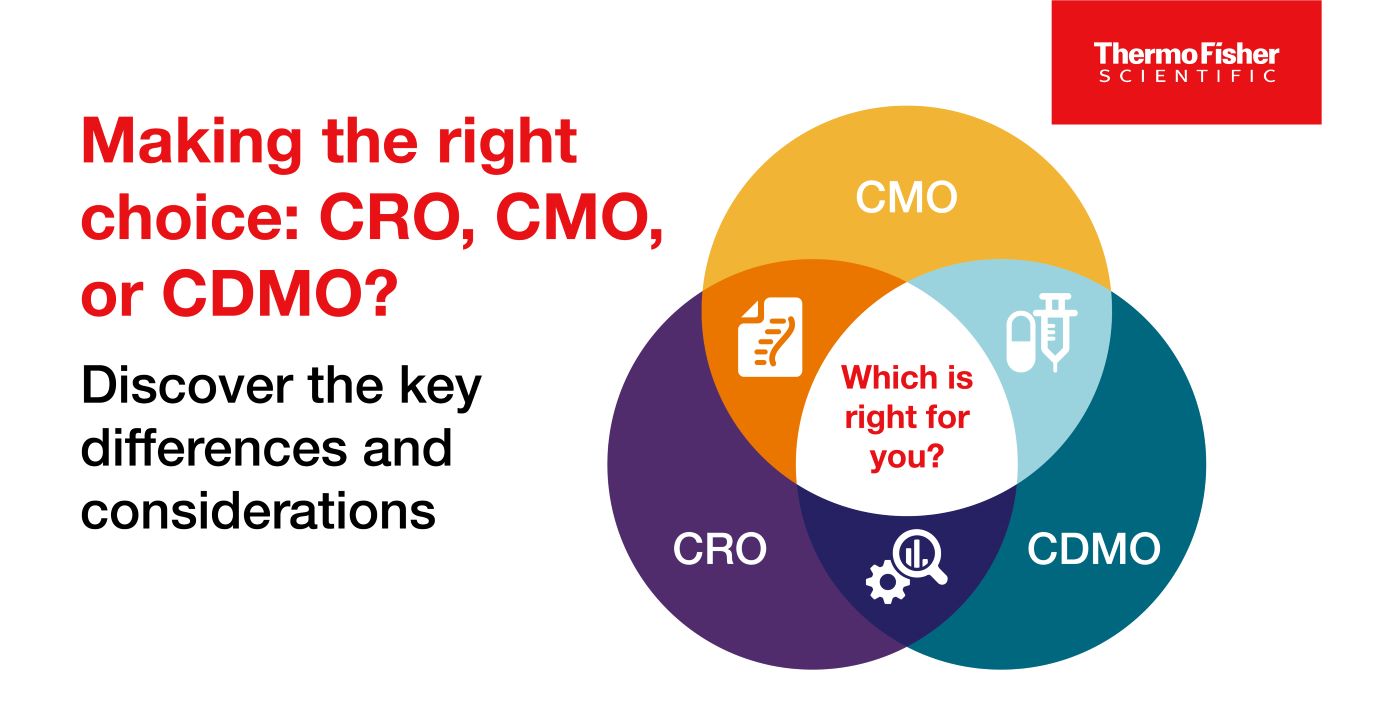
Blog post
CRO / CMO / CDMO 차이점
CRO, CMO, CDMO는 모두 약물 개발 및 제조와 관련된 서비스를 제공합니다. 자료에서는 이 세 조직과 서비스의 차이점을 설명합니다.
9 minute read

Blog post
세포 및 유전자 치료제 개발 - 환자 중심의 접근 방식
블로그 포스트를 통해 세포 유전자 치료제 개발의 환자 중심적 접근 방식에 대해 알아보세요.
8 minute read

Blog post
통합된 업무 프로세스를 통한 디지털 업무 환경 조성
안정성과 경험이 중요한 물리적 업무 환경과는 대조적으로, 디지털 업무 환경은 혁신과 연결성에 초점을 맞춥니다. 자료를 통해 디지털 업무 환경 조성에 대한 자세한 내용을 확인하세요.
4 minute read

Blog post
바이러스 벡터 상용화 – Part 1: 기술 이전 프로세스
기술 이전으로 바이러스 벡터 개발과 생산 규모를 확장하고, 백신 및 유전자 치료제 상용화 속도를 높이는 방법을 확인하세요.
9 minute read

Blog post
Keeping your information safe: IP and data protections in China and across the globe
Experts at Thermo Fisher discuss rigorous contractual, project team, and data protections that are in place to keep customers’ intellectual property and confidential information secure.
12 minute read

Blog post
Manufacturing in China for China: Navigating the regulatory landscape
Experts at Thermo Fisher provide insight into the registration and approval pathway for developing and marketing biologic therapies in China for the domestic market.
12 minute read
Blog post
Viral vector commercialization – Part 3: Specialized regulatory support
Find detailed regulatory considerations when preparing viral vectors for commercialization and best practices to address them.
7 minute read

Blog post
Ensuring Quality Consistency at Hangzhou Site
Find Q&A insights from experts at Thermo Fisher on the quality systems framework that guides quality implementation at our new Hangzhou site.
12 minute read

Blog post
Patheon Translational Services Advance Cell and Gene Therapies from Research to Clinical Trials
Learn how our translational research services, housed in our San Diego facility, can take cell and gene therapies from preclinical to clinical.
6 minute read

Blog post
Top tips for providing the right amount of detail in first-in-human common technical documents
In the early-development stage, little may be known about a drug’s characteristics. What’s more, drug processes and formulations frequently evolve as more information emerges following testing and trials. Learn more.
5 minute read

Blog post
Gaining a deeper understanding of your API
As active pharmaceutical ingredients (APIs) become increasingly complex, they pose potential formulation problems that can extend timelines and explode budgets. Read this blog to learn more.
5 minute read
Blog post
EU and US regulations: What’s coming for cell and gene therapies?
Cell and gene therapy (CGT) developers today face an added challenge in their quest to bring a product through clinical trials and to the market. Read this blog to learn more.
7 minute read
Blog post
Trends in mRNA therapeutics: Pandemic learnings for a pathway to success
The rapid advancement of the Pfizer-BioNTech and Moderna messenger RNA–based COVID-19 vaccines from lab to clinic—with development taking less than one year—has validated the...
5 minute read
Blog post
Choosing a CDMO for mRNA success: Five CDMO characteristics needed
The promise of mRNA technologies has been clearly demonstrated during the COVID-19 pandemic, with vaccines reaching the market in record time. The vital role...
7 minute read

Blog post
Moving from vials to prefilled syringes for vaccines: Three key success factors
As pharmaceutical companies become more patient-centric and self-administration of injectable drugs continues to increase, the market for drug products in prefilled syringes is forecast to grow, reaching $9.53 billion by 2026.
4 minute read

Blog post
Choosing a CDMO Who is a True Bioproduction Expert
With the number of CDMO’s rising in the biologics manufacturing industry, it can be challenging for new and emerging biopharmaceutical companies to determine which CDMO is right for them.
8 minute read

Blog post
Introducing an Expanded Packaging Service for Specialty Products
Developing a specialty drug for a complex or rare disease is an achievement worthy of celebration.
2 minute read

Blog post
Top Tips on How to Manage Clinical Label Translation and Regulatory Requirements
Wouldn’t it be nice to find a way to stay on top of label translation and regulatory requirements for every country included in your clinical trial?
2 minute read

Blog post
Is Your Supply Chain Bulletproof?
Before you respond, think hurricanes, Nor’easters and tsunamis. Earthquakes, typhoons and volcanic eruptions. Civil unrest and war. Terrorism. A pandemic virus.
2 minute read

Blog post
Myths & Facts about Ancillaries
It’s a fact that ancillary supplies are frequently perceived as less important than study drug.
2 minute read

Blog post
The Four Stages of Equipment Qualification
As discussed in my previous blog, qualification is the process of establishing documented evidence that a specific equipment, facility or system are fit and ready for their intended use.
3 minute read

Blog post
Navigating Cell & Gene Therapy Regulations: How Does Your CDMO Match Up?
Whether you are a large or new and emerging biotech company, many companies find themselves lacking the internal resources and/or expertise to properly support regulatory submissions.
7 minute read

Blog post
Prefilled Syringes: Three Pain Points You May Not Have Considered
As pharmaceutical companies look to become more patient-centric, certain drug products come to the forefront to support that effort.
5 minute read

Blog post
Work Smarter, Not Harder: Accelerating Your Biologics Development and Commercialization
Over the past decade, the biologics industry has seen double digit growth and an overall increase of market share.
5 minute read

Blog post
How Decentralized Clinical Trials Enhance Patient-Centricity in the Age of COVID-19
As COVID-19 continues to change how we do business in the biopharmaceutical industry, it’s important to not lose sight of why we do what we do: improving and saving patient lives.
5 minute read

Blog post
A Day in the Life of a Viral Vector Partner
When it comes to a viral vector Contract and Development Manufacturing Organization (CDMO), what sort of qualities should they possess?
9 minute read

Blog post
Continuous Manufacturing: An Efficient Way to Produce OSD Drugs
When Henry Ford revolutionized manufacturing practices back in 1913 in Highland Park, MI, his main goal was simple—to make the best possible product in the most efficient and cost-effective manner. Ford’s focus on bettering the “flow” of manufacturing to enable workers/technology to work smarter and reduce waste of raw materials, changed manufacturing principles forever.
6 minute read

Blog post
COVID-19’s Silver Lining: Accelerated Vaccine Development
Vaccine development is a lengthy process—it is expensive, attrition is high, and to get a licensed vaccine to everyone, it takes multiple candidate iterations. Vaccine development for pandemics and epidemics is risky, and due to the novel nature of viruses, certain unknown factors can derail a vaccine program.
6 minute read

Blog post
Simplifying Your API's Development Journey
The primary way new drugs are developed is changing. Historically, pharma companies—particularly large pharma companies—have developed APIs in-house.
7 minute read

Blog post
Considerations and roadblocks that stifle orphan drug development
According to the US Food and Drug Administration (FDA), “2020 was a record-breaking year in terms of the number of orphan drug designation and rare pediatric disease designation requests submitted to the Office of Orphan Products Development.”
6 minute read

Blog post
Break Through the OTC Noise
It’s no secret—consumers have a vast range of OTC options at their favorite in-store or online retailer. Everything from tablets and capsules, to syrups—consumers have more options than ever in the OTC jungle.
8 minute read

Blog post
Taking Your API to the Next Level: Three Steps to Consider Before Outsourcing
With outsourcing API development becoming more common, we see a rise of multiple competing Contract Development Manufacturing Organizations (CDMOs) as potential development partners.
10 minute read

Blog post
Is it Time to Start Thinking about Packaging?
If this headline caught your eye, it may be because you’ve received promising pre-clinical results (Congratulations!) and you’re starting to think about planning your next steps.
2 minute read

Blog post
Time to embrace electronic labels? Potential labeling solutions under the new EU Clinical Trial Regulation
The new EU Clinical Trial Regulation (CTR) is intended to simplify clinical trial administration and create a more welcoming climate for pharmaceutical companies that operate in Europe.
5 minute read

Blog post
Navigating the Complexities of Process Performance Qualification
Method qualification is monumentally important before process performance qualification (PPQ). This early assessment of your method’s performance characteristics is critical as it pertains to method validation and its parameters such as precision, accuracy, and linearity.
5 minute read


