라이브러리
다양한 자료를 통해 업계 인사이트 및 당사 서비스에 대해 자세히 알아보세요.
당사는 분야별 전문가를 보유하고 있으며 약물 생산 및 임상시험 업계 전반에서 기술 및 과학 전문가들과 함께 분자부터 약품까지 모든 개발 단계를 설명하는 whitepaper, 기사, 웨비나 등 다양한 자료를 제공합니다.
메뉴 버튼을 사용하여 검색 범위를 지정하거나 아래 필터를 사용하여 카테고리, 분야별 전문가 또는 콘텐츠 유형별로 전체 라이브러리를 검색할 수 있습니다.
문의 사항이 있으신가요? 문의하기
관심 분야를 선택해 주세요.

Event
Webinar
AI/ML 기반 in silico 예측 모델링을 활용한 저분자 약물 개발 전략 웨비나
2026/2/24(화) 오전 10시 AI/ML 기반 in silico 예측 모델링을 활용한 저분자 약물 개발 전략을 논의하는 웨비나가 개최됩니다.

Event
Tradeshow
DCAT Week 2026
Meet with us at DCAT to learn how our connected CDMO, CRO, and clinical supply services support sponsors at every stage of the drug development journey.

Event
Conference
ISCR 19th Annual Conference
Visit our booth at the ISCR 19th Annual Conference in New Delhi and explore how our comprehensive, global clinical trial services and solutions can help support the success of your next study.

Infographic
Autoinjector, pen, and prefilled syringe capabilities
Download this interactive infographic to explore how our scale- and platform-flexible autoinjector, pen, and prefilled syringe capabilities can accelerate your biologic drug development supply chain.

Blog post
Enabling flexibility and scientific innovation in oral solid dose development
A strategic CDMO partnership enabled a clinical-stage pharmaceutical company to navigate the complexities of early drug development, advancing formulation strategies, adapting to shifting priorities, and ensuring a scalable path to clinical trials.
12 minute read

Event
Tradeshow
Clinical Trial Supply Europe 2026
Connect with our experts at CTS and discover how our integrated CDMO and CRO offerings and our strategic clinical supply services can support your trial needs.

Whitepaper
Redefining OSD Development Through Foresight and Innovation
Examine how technology, global networks, customized services and regulatory expertise work together to overcome challenges of oral drug development.

Blog post
The real challenge of biologics scale-up isn’t capacity—it’s demand
Most biologics never reach the production volumes that justify large-scale stainless-steel capacity. The real challenge isn’t building enough—it’s knowing how much will truly be needed, and when. Here’s why flexibility in scaling is becoming one of biologics manufacturing’s most strategic advantages.
16 minute read

Webinar
Scaling smart: Overcoming the top roadblocks in biologic drug manufacturing
This on-demand webinar explores how a unified, global CDMO model can help biologic drug developers overcome the most pressing challenges in clinical and commercial manufacturing.

Webinar
Accelerate with confidence: Research-based insights to guide CDMO–CRO partnership decisions
This webinar shares key findings from a Tufts study that quantifies both the financial returns and risk-adjusted value of working with an integrated CDMO, CRO, and clinical supply partner.

Blog post
How to Achieve Late-Phase Success in OSD Development
Learn how to achieve late-phase success in oral solid dose scale-up with data-driven strategies, risk mitigation, and the right CDMO partnership.
11 minute read
Infographic
Path to IND for biologics: Go from DNA to drug product in as few as 9 months
See how Path to IND for biologics can save time while maintaining quality. Learn the key steps that compress timelines, including the use of our CHO K1 cell line with transposase technology.

Webinar
From model to molecule: Combining AI and experimental strategies to transform drug development
This on-demand webinar explores real-world case studies demonstrating how the integration of computational and experimental strategies can help overcome drug development challenges.

Case Study
Accelerator™ Drug Development: Integrated governance drives execution across global clinical research program
This case study highlights how one biopharma sponsor leveraged Accelerator™ Drug Development to regain control of its clinical research program and deliver results across their portfolio.

Infographic
Unlocking ROI and Efficiency in Drug Development
A data-driven look at an integrated approach to drug development.

Event
Tradeshow
Biotech Outsourcing Strategies (BOS) Manchester 2025
Visit Thermo Fisher Scientific at booth #47 to learn how Accelerator™ Drug Development can streamline and speed up early-phase OSD development for small molecules and biologics.

Event
Tradeshow
Clinical Outsourcing Group (COG) Europe 2025
Let’s connect at COG Europe 2025 in November to discuss how our integrated CDMO and CRO solutions, including clinical trial supply services, can help get your treatments to patients faster.

Event
Conference
Clinical Trial Supply East Coast 2025
Visit booth #50 to explore how our integrated CDMO and CRO solutions, including clinical supply services, can help you get treatments to patients faster.

Event
Conference
AAPS 2025 PharmSci360
Join us at AAPS 2025 to explore the modern technologies we utilize to enhance oral solid dose and small molecule drug development processes.

Event
Conference
AusBiotech International Conference 2025
Visit booth #87 at AusBiotech International Conference 2025 to learn how our end-to-end CDMO services and solutions can help speed up your drug development and manufacturing journey.

Blog post
Discussing the Future of Biotech
Explore expert insight into what trends are shaping the biotech industry, including innovations, sustainability, AI, and growth in a dynamic landscape.
10 minute read

Case Study
Accelerator™ Drug Development: Streamlining preclinical pathways for a faster transition to First-in-Human trials
Download the case study to see how one biotech company saved $1M and shortened development timelines by 12 months with Thermo Fisher Scientific’s integrated CDMO and CRO solutions.

Blog post
Accelerate drug development by embracing an integrated approach
Pharmaceutical companies face significant challenges with rising R&D costs and extended development timelines. Embracing an integrated CDMO and CRO approach that combines drug substance, drug product, clinical manufacturing, clinical research, and clinical supply chain management into one streamlined service can simplify complexity, enhance efficiency, and reduce risks, ultimately accelerating the journey from lab to market.
12 minute read
Webinar
Accelerating biologics: From final DNA to Phase I in under 9 months
Originally presented at BIO International 2025, this webinar explores how Path to IND helps biotech companies move from DNA to first-in-human Phase I clinical trials in as little as nine months.*

Event
Conference
Festival of Biologics Basel 2025
Visit us at Festival of Biologics to learn about our innovative technology powering process development and late-stage biologics production and manufacturing.
Blog post
Expert perspective: How Thermo Fisher Scientific’s packaging and labeling innovations simplify biotech clinical trials
Find expert insight on how innovative clinical packaging and labeling solutions can streamline processes, enhance efficiency, compliance and overall success.
15 minute read

Event
Conference
2025 Cell & Gene Meeting on the Mesa
Join us at Meeting on the Mesa to explore how our integrated CDMO and CRO solutions for advanced therapies can support the unique needs of your program.

eBook
Accelerate drug development with innovative 360˚ CDMO and CRO solutions: A comprehensive guide to streamlining drug development and reducing time to market
This eBook outlines solutions to help maximize resources and minimize risks across all phases of drug development, supporting each biotech or pharma company’s unique journey to market.

Webinar
Stop outsourcing, start partnering: Redefining value in global clinical trial execution
11 studies. 51 countries. One team. Learn how reframing the CDMO as a value-driving partner helped NewAmsterdam Pharma’s lean team achieve remarkable results.

Webinar
Accelerating biologics development with strategies for success
This webinar explores how drug developers can deliver innovative biologics quickly, all while navigating regulatory requirements, clinical trials, production scale-up, and quality control.

Event
Conference
ISPE Singapore 2025
Meet Thermo Fisher Scientific at ISPE Singapore 2025, August 27–29. Visit booth #244 or book a 1:1 meeting to explore how our CDMO capabilities support pharma and biotech across APAC and beyond.

Event
Tradeshow
Clinical Trial Supply West Coast 2025
Visit booth #15 to explore how our integrated CDMO and CRO solutions, including clinical supply services, can help you get treatments to patients faster.

Event
Tradeshow
GCSG 2025 European Knowledge Forum
Join Thermo Fisher Scientific at the GCSG 2025 European Knowledge Forum from October 14–16, 2025, to learn more about our integrated clinical supply, CDMO, and CRO solutions.

Fact sheet
빠른 임상 1상 진입을 위한 Path to IND 바이오 약물 개발 가속 솔루션
Path to IND for biologics 플랫폼을 이용하면 고분자 약물을 transfection 시작 후 최단 9개월만에 FIH 임상에 투여할 수 있도록 지원합니다

초기 단계 경구제 제형 개발의 5가지 숨은 리스크
초기 단계 경구제 개발에는 장기적인 성공에 영향을 미칠 수 있는 다양한 숨은 리스크가 존재합니다. API의 복잡성부터 생산 규모 확대 가능성, 규제 대응 준비까지, 신중한 계획 수립은 예상치 못한 차질을 줄이고 개발 프로그램을 원활히 진행하는데 도움이 됩니다.
12 minute read

의약품 개발에서 ROI와 효율성 극대화
새로운 연구에 따르면, 제조, 임상 연구, 물류 서비스를 단일 파트너로 통합하면
의약품 개발 과정의 복잡성을 줄이고, 실행을 간소화하며, 상당한 ROI를 실현할 수 있는 것으로 나타났습니다.

바이오의약품을 위한 세포주 개발: 안정성과 수율 향상
세포주 개발은 바이오의약품 제조에서 매우 중요한 과정으로 공정 효율성, 생산 규모 확장성, 제품 안정성에 큰 영향을 미칩니다. 최근 CHO K1 세포주 엔지니어링의 발전으로 인해 수율이 향상되고 유전자 안정성이 개선되며 IND 제출까지의 기간도 단축되고 있습니다.
15 minute read
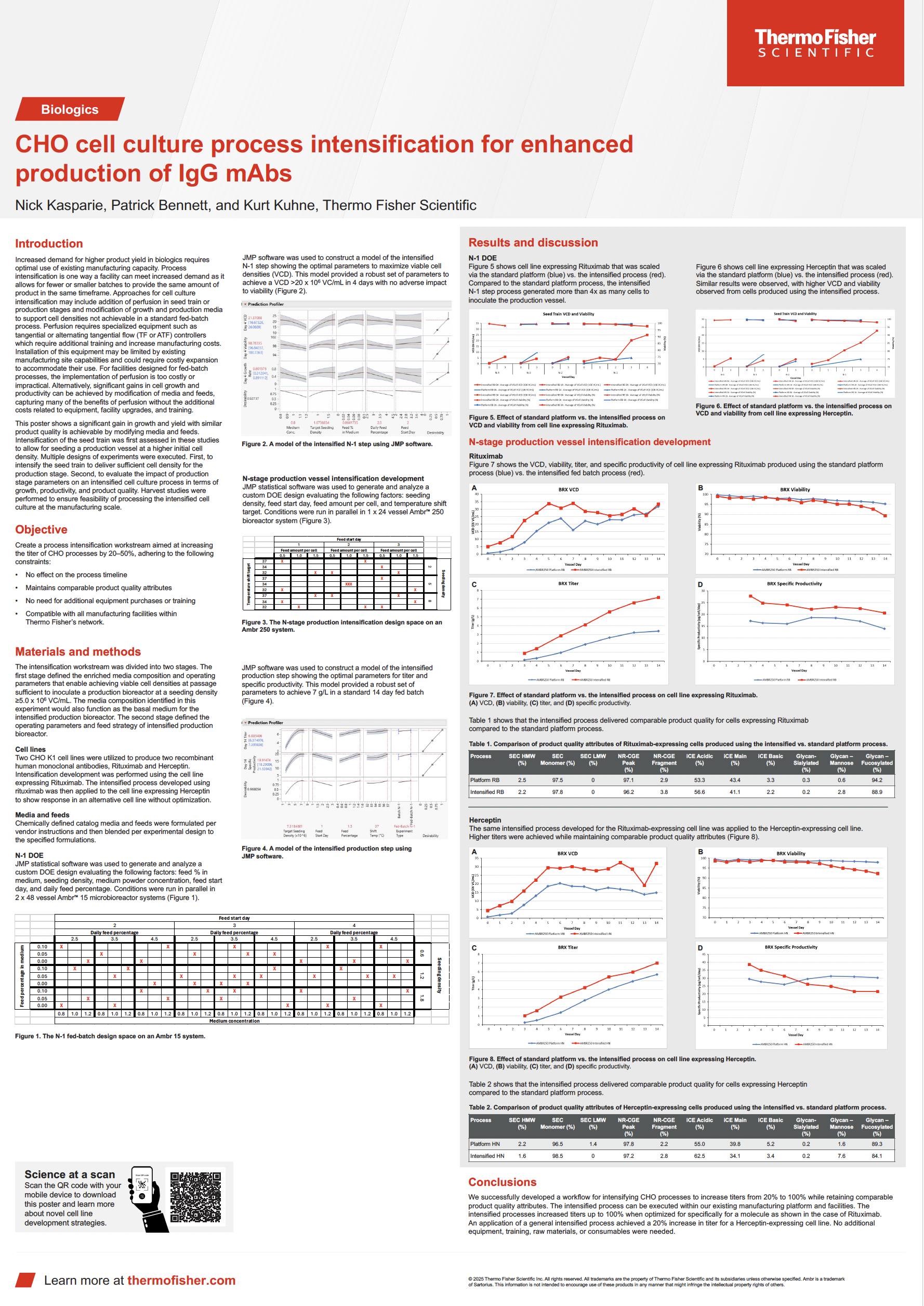
IgG mAbs 생산 증대를 위한 CHO K1 세포 배양 공정 집약화 (Process Intensification)
기존 제조 플랫폼과 시설을 활용하여 CHO K1 세포주로 품질 저하 없이 titer를 80%까지 향상시킨 방법을 확인하실 수 있습니다.

Understanding large molecule drugs
이 블로그에서는 바이오 의약품에 대해 심층적으로 살펴보며, 그 주요 특성, 장점과 과제, 그리고 제약 산업에서의 미래를 다룹니다.
18 minute read

eBook
Global reach, biotech speed: 임상용 패키징 및 라벨링 서비스
바이오의약품 임상용 패키징 및 라벨링 파트너에게 반드시 필요한 세 가지 핵심 역량을 확인하고, 당사의 차별화된 솔루션이 귀사의 다음 임상시험을 어떻게 지원할 수 있는지 알아보세요.

Webinar
Securing your samples: 바이오 샘플 보관 전략
이 웨비나에서는 바이오의약품 및 세포·유전자 치료제를 위한 초저온 보관과 관리의 중요성을 살펴보고, 적합한 CDMO 파트너가 제품의 품질과 안정성을 어떻게 보호할 수 있는지 설명합니다.

Event
Tradeshow
BIO Taiwan 2025
Connect with us July 23-27 in Taiwan, to explore pharmaceutical supply chain hot topics and discuss how we can accelerate your journey from molecule to medicine to market.

Event
Conference
CRS 2025 Annual Meeting and Exposition
Connect with us at CRS to explore our specialized expertise and full range of controlled release technologies and formulation services.

Event
Tradeshow
Interphex Japan 2025
Connect with Thermo Fisher Scientific at Interphex Japan 2025 from July 9 to 11 to explore how we accelerate your drug development journey to global market.
Seminar
Seminar
Path to IND: 신속한 글로벌 임상 진입과 규제 변화 대응 전략 세미나
신속한 임상 진입과 글로벌 성공을 위한 규제 변화 대응 전략을 논의하는 Patheon 바이오 세미나에 초대합니다.

Webinar
Unlocking the potential: 펩타이드·단백질 경구 투여의 도전 과제와 기회
펩타이드 단백질 약물 경구 투여의 주요 과제와 약물 개발 가속화를 위한 전략을 확인하세요.

Event
Conference
BOS Basel 2025
Visit us at booth #57 at BOS Basel 2025 from June 11–12, 2025 in Basel, Switzerland to discover how Accelerator™ Drug Development can drive early development for OSD.

Event
Tradeshow
BIO International Convention
Join us in Boston at BIO International to explore how we can support your biologics program from early decisions through IND readiness and beyond.

eBook
Optimizing clinical trial logistics for success
This comprehensive guide discusses the complexities of clinical trial logistics. Learn how Thermo Fisher Scientific’s Total Transportation Management can help ensure timely and secure delivery of clinical trial materials worldwide.
From clinical to commercial: Streamlining cold chain logistics for advanced therapies
Discover how Thermo Fisher Scientific can optimize cold chain logistics for your advanced cell and gene therapy project.

Webinar
Assessing the application of standardized processes in cell and gene therapy development and manufacturing
This webinar features experts in cell and gene therapy (CGT) development, regulatory affairs, and CMC strategy, offering guidance on assessing the risks and rewards of standardized processes.

Event
Webinar
펩타이드 단백질 약물 경구 투여 웨비나
2025/5/27(화) 오전 10:30 펩타이드, 단백질 약물 경구 투여의 주요 과제와 약물 개발 가속화를 위한 전략을 논의하는 웨비나가 개최됩니다.

Event
Tradeshow
Bio Korea 2025
Connect with Thermo Fisher Scientific at Bio Korea 2025 from May 7–9 to explore our end-to-end CDMO capabilities for large molecule development and manufacturing projects.

Event
Conference
ISBER 2025 Annual Conference
Let’s connect at ISBER 2025 to explore our cold and ultracold supply chain services and solutions, including our comprehensive biobanking capabilities. Visit our team at booths #4 and #5.

Event
Conference
Festival of Biologics 2025
Visit Thermo Fisher Scientific at booth #773 from April 23 – 24 to discover our cutting-edge new platform that accelerates biologics drug development like never before: Path to IND for biologics.

Event
Tradeshow
Bio Korea 2025
5/7부터 3일간 개최되는 바이오코리아 2025에 초대합니다. 당사 부스를 방문하고 글로벌 CDMO와의 전략적 파트너십에 대해서 자세히 알아보세요.

Event
Tradeshow
CPHI Japan 2025
Join us in Tokyo, Japan, from April 9–11 to dive into key pharmaceutical supply chain topics and explore ways to accelerate your path from molecule to medicine to market.

Event
Conference
ASGCT 28th Annual Meeting
Let's connect at ASGCT 2025 to discuss our CDMO capabilities for advanced therapies, including the development and manufacturing of viral vectors, cell therapies, and mRNA. Visit booth #1527.

Event
Conference
ISCT 2025
Let’s connect at the International Society for Cell & Gene Therapy (ISCT) Conference in New Orleans, LA, from May 7 – 10 to discuss our comprehensive CDMO capabilities for advanced therapies.

Seminar
Seminar
AI and digital solutions: Advancing drug development and clinical trials
AI 기술 기반 신약 개발 전략 및 임상시험의 디지털 혁신 세미나에 초대합니다.

Tech transfer, part 1: 의약품 생산 불확실성 극복하기 - 기술 이전의 핵심 역할
효율적인 기술 이전은 제품 품질 유지, 지적 재산 보호, 비용 관리, 및 생산 규모 확대를 지원하여, 제약 기업이 새로운 기회와 도전에 효과적으로 대응하고 경쟁력을 유지하며, 환자에게 지속적인 의약품 공급을 보장할 수 있도록 합니다.
15 minute read

5,000L 싱글유즈 바이오리액터(Single-use bioreactor)의 바이오의약품 생산 혜택
바이오의약품 생산에서 생산 용량 평가 방법, 각 신약 개발 단계에서 싱글유즈 바이오리액터를 활용하는 이점에 대해 소개합니다.
Article
초기 개발부터 상업화 성공까지 - 유전자 치료제 개발의 모든 단계 지원을 위한 바이러스 벡터 서비스
유전자 치료제 개발의 서로 다른 단계에 위치한 두 기업, NysnoBio와 bluebird bio가 각각 필요로 했던 파트너십의 유형과, 이들이 혁신적인 치료제를 환자에게 전달하기 위해 어떤 지원을 받았는지를 살펴봅니다.

Webinar
Rapid Development Framework™ - 세포 유전자 치료제 개발 생산 가속 플랫폼
Rapid Development Framework™가 세포 유전자 치료제 생산 공정 및 분석을 최적화하여 개발 및 생산 일정을 단축하는 동시에, 각 치료제의 고유한 요구사항에 유연하게 대응하는 방법을 알아보세요.

Event
Tradeshow
NextGen Biomed 2025
Let’s connect at NextGen Biomed 2025 in London to discuss how our CDMO and CRO solutions support biotech companies of all sizes and stages in advancing their life-changing treatments.

Event
Tradeshow
Clinical Outsourcing Group (COG) Nordics 2025
Let’s connect at COG Nordics from April 1-2, 2025, to discuss how our integrated CDMO and CRO solutions, including clinical supply services, can help you get treatments to patients faster.

Event
Tradeshow
GCSG 2025 US Conference
Join Thermo Fisher Scientific at GCSG 2025 from April 27–30 to explore our comprehensive clinical trial supply capabilities and see how we can help streamline and support your supply chain needs.

Optimizing clinical supply management with a one-team approach
Download our case study to explore how our CDMO and CRO services helped NewAmsterdam Pharma deliver investigational medicinal products for over 12,000 patients across 835 clinical sites.

Event
Tradeshow
Advanced Therapies Week 2025
Visit booth #541 at Advanced Therapies Week 2025 from January 20-23 in Dallas, Texas, to discover our CDMO services and solutions for advanced therapies, including cell and gene therapies.

Seminar
Seminar
Addressing challenges in steriles product development with a patient-centric approach
Sterile drug product 세미나에 초대합니다.

Blog post
Regulatory landscape in Europe: Key advice for meeting post-Brexit Qualified Person requirements
The United Kingdom’s (UK) departure from the European Union (EU) has added a layer of complexity to the clinical trial supply chain in Europe above and beyond COVID-related disruptions.
(4 minute read)

Event
Conference
Festival of Biologics 2024
Join us at Festival of Biologics to meet our experts and see how our integrated CDMO services can support your biologics needs.

Event
Conference
12th Annual Outsourcing in Clinical Trials Nordics 2024
Meet with Thermo Fisher Scientific at the 12th Annual Outsourcing in Clinical Trials Nordics 2024 to discover our CDMO services and solutions, including comprehensive capabilities for clinical trials.

Event
Event
BIO-Europe 2024
Visit booth #100 at BIO-Europe 2024 from November 4-6 in Stockholm, Sweden, to discover our CDMO and CRO services and solutions for large molecule drugs, including biologics.

Event
Tradeshow
Bioplus Interphex Korea 2024
Thermo Fisher Scientific이 7월 10일~12일 코엑스에서 개최되는 BIX 2024에 참가합니다.

Event
Tradeshow
Bio Korea 2024
5/8부터 3일간 개최되는 바이오코리아 2024에 초대합니다. 당사 부스를 방문하고 글로벌 CDMO와의 전략적 파트너십에 대해서 자세히 알아보세요.

Blog post
생체시료 저장소 구축의 장단점 비교
생체시료 저장소는 생물학적 물질을 수집, 보존, 활용하는 데 중요한 역할을 합니다. 이를 사내에 구축하는 것과 구입하는 것 중 어떤 것이 합리적일까요?
8 minute read

Fact sheet
저분자의약품 Quick to Clinic™
저분자 경구제용 Quick to Clinic 프로그램은 in-silico modeling, API, 고체 화학, 분석법 및 제형 개발을 지원합니다.
Infographic
빠른 IND/IMPD를 위한 5가지 방법
품질을 유지하면서 향후 상업화 목표에 차질 없이 IND/IMPD에 빠르게 도달하는 방법은 무엇일까요? 인포그래픽을 통해 초기 개발 프로세스를 최적화하는 방법에 대한 당사 전문가들의 제안을 확인하세요.

eBook
환자, 스폰서, 시험자에게 성공적인 분산형 임상시험의 방법
Direct-to-Patient 서비스가 분산형 임상시험의 성공에 필수적인 이유와 이를 활용하여 의약품 개발을 개선하는 방법을 설명합니다.

Webinar
EU 규제 환경 탐색: 의약품 개발, 치료 혁신 및 시장 접근성 가속화 전략
총 3부로 구성된 이 웨비나 시리즈에서는 성공적인 인허가를 위해 기업이 의약품 개발 과정에서 염두에 두어야 할 주요 영역을 다룹니다.
Article
전문가 기고: 저분자 API 개발 및 인허가 간소화
API 개발 단계에서 규제 요건을 충족하기 위한 주요 과제, 이를 극복하기 위한 권장 사항, 신뢰할 수 있는 CDMO와의 파트너십이 어떻게 여정에 큰 변화를 가져올 수 있는지에 대한 전문가의 글을 확인하세요.

Fact sheet
Qualified Person (QP) 서비스
자료를 통해 에어로졸, 생물학적 제제, 크림, 액체, 연고, 고체, 무균제제 및 새로운 약물 전달 시스템을 포함한 다양한 제형에 대한 폭넓은 경험을 보유한 당사의 QP 팀에 대해 알아보세요.

유럽의 규제 환경: EU 및 영국에서 의약품 공급을 위한 새로운 QP 요건 이해하기
EU와 영국에서 임상시험 및 상업화를 추진하는 의약품 개발자는 새로운 환경에서 유럽 GMP 요건과 EU 및 영국 QP의 역할과 책임에 대한 깊은 이해가 필요합니다.

Event
Tradeshow
25th Annual Clinical Trial Supply Europe 2024
Visit booth #65 to meet with industry-leading experts from Thermo Fisher Scientific and explore our end-to-end global clinical supply chain services.

Event
Tradeshow
DCAT Week 2024
Join us at DCAT Week in New York City where will be discussing the elements of a trusted partnership and what’s needed to ensure your project goes smoothly.

Event
Tradeshow
Indian Society for Clinical Research — 17th Annual Conference
Visit our booth at ISCR 2024 to learn how our comprehensive CDMO services and solutions can support your clinical trial management and supply chain logistics needs.

Article
[인터뷰] 써모 피셔 사이언티픽 "호주 파테온 CDMO, 韓 신약 세계화 교두보"
호주 브리즈번 바이오 DS 생산시설 대표의 인터뷰를 통해 당사 브리즈번 사이트의 역량과 한국 기업의 바이오 의약품 생산 사례 등에 대해 자세히 알아보세요.

Fact sheet
대조약 및 병용약 소싱 서비스
당사의 대조약 전문팀(Comparator Center of Excellence Team)이 각 국가 및 전 세계에서 약물을 확보하기 위한 가장 비용 효과적인 전략을 개발하는 데 어떻게 도움을 드릴 수 있는지 확인하세요.

Infographic
바이러스 벡터 분석 특성화 모범 사례
인포그래픽에서 불순물 분석과 중요품질특성 정의 시 문제 극복을 위한 전략을 비롯하여 바이러스 벡터 분석 특성화를 성공적으로 수행하는 팁과 고려할 점에 대해 알아보세요.

Fact sheet
통합 운송 관리(TTM) 서비스
당사의 통합 운송 관리(Total Transportation Management) 서비스는 국내외에서 모든 유형의 생명과학 선적물을 이동하는 데 필요한 복잡한 공급망 프로세스를 관리합니다.

Blog post
바이러스 벡터 상용화 – Part 2: 프로세스 밸리데이션 주기 모범 사례
바이러스 벡터의 안전성, 유효성 및 품질을 보장하기 위한 다양한 평가 및 시험을 포함하는 바이러스 벡터 공정 밸리데이션 주기에 대해 자세히 알아보세요.
11 minute read

Blog post
CDMO 파트너를 선택할 시 고려해야 할 7가지
CDMO가 제약사와 협력하는 방법에 대해 알아보고 기업이 CDMO 파트너를 선정할 때 고려해야 할 주요 고려사항에 대해 확인하세요.
9 minute read
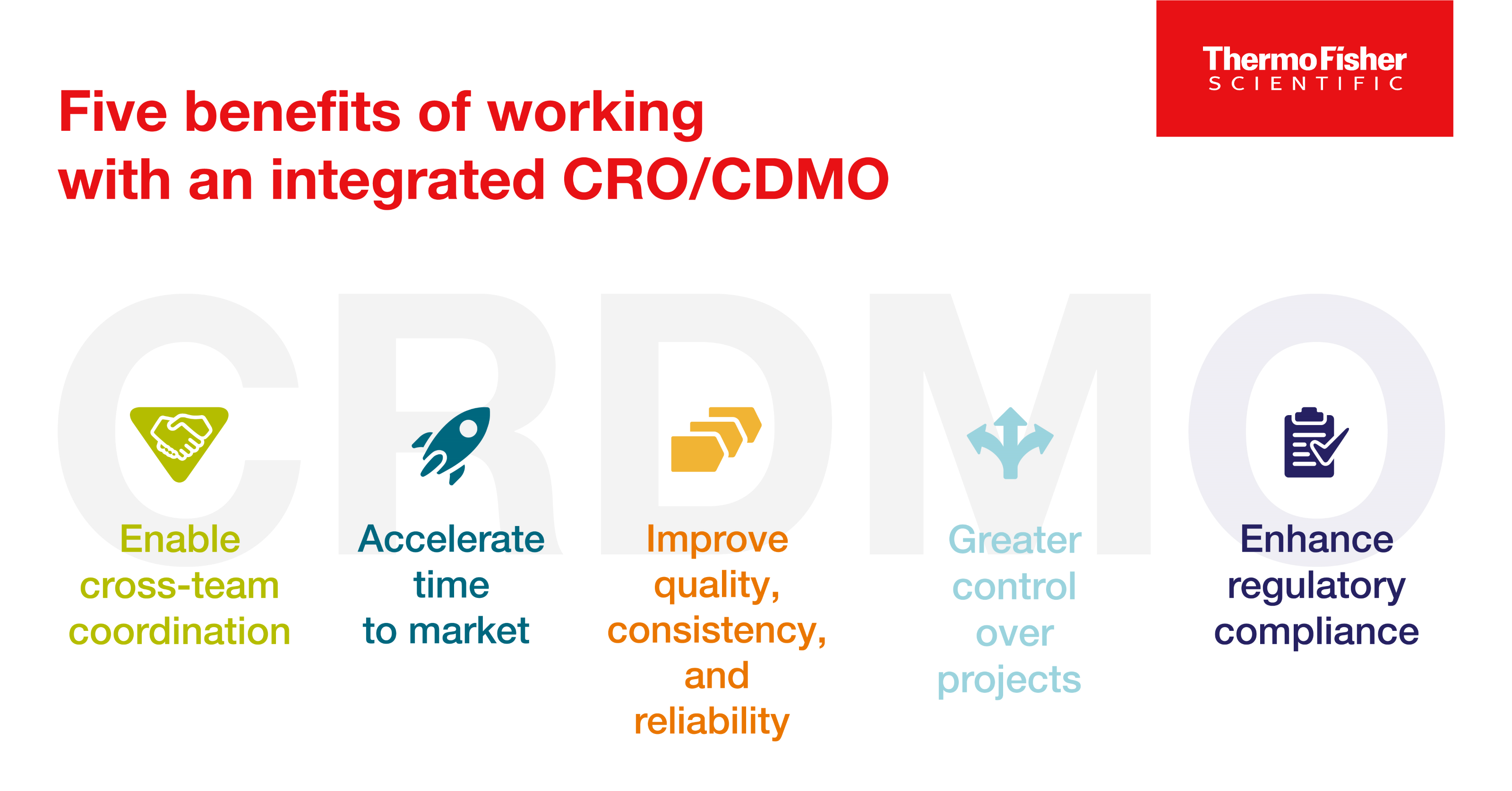
Blog post
CRDMO - CRO/CDMO 통합을 통한 의약품 개발의 재구성
의약품 개발사는 통합 CRO/CDMO와 협력함으로써 여러 아웃소싱 파트너와 협력해야 하는 복잡함 없이 새로운 치료법을 시장에 출시하는 과정을 보다 효율적으로 탐색할 수 있습니다. 통합 CRO/CDMO와 협력할 때 얻을 수 있는 이점에 대해 알아보세요.
9 minute read

Video
자가 및 동종 세포치료제 - 과학, 제조 및 규제 고려사항 탐색하기
이 자료는 동종 세포치료제 및 자가 세포치료제 관련된 복잡한 역학 관계를 다루고 업계의 현재 및 미래 전망에 대한 통찰력을 제공합니다. 제조 및 물류, 규제 환경 및 CMC 요건, 표준화 역할에 대한 모범 사례도 확인하실 수 있습니다.
초기 및 후기 단계 공정의 조화를 통한 약물 개발 가속화 전략
현행 GMP 플랫폼을 반영하는 기술과 공정을 사용하여 발견 연구를 위한 AAV와 LV 벡터 제조를 위해 Thermo Fisher Scientific에서 수립한 효율적인 접근법을 제시합니다.

Whitepaper
대조약 소싱 - 제네릭 및 바이오시밀러에 대한 시장 접근을 가속화하기 위한 볼라 조항 활용하기
Thermo Fisher의 대조약 팀은 볼라 조항을 활용하는 데 필요한 전문성을 갖추고 있으며 고객의 제네릭/바이오시밀러 약물을 가속화할 수 있도록 지원합니다.
Whitepaper
항암제 개발 환경 변화 - 환자의 생명을 구하는 새로운 치료제 개발
항암제는 가장 빠르게 성장하고 연구가 활성화된 신약 개발 분야입니다. 혁신적인 기술을 도입하면 임상과 상업화를 위한 약물 개발을 가속할 수 있습니다.

Webinar
mRNA 치료제 상업화에 대한 업계의 도전과제 및 해결방안
COVID-19 팬데믹 기간 동안 업계는 mRNA 기반 백신의 신속한 개발과 승인을 목격하며 종양학, HIV, 희귀 질환을 위한 치료제를 위한 mRNA 기술 적용에 높은 관심을 보이기 시작했습니다. mRNA의 생산 속도와 유연성은 매력적이지만, 완전한 잠재력을 실현하고 그 사용을 더욱 확장하기 위해서는 여전히 몇 가지 업계 과제를 해결해야 합니다.

Fact sheet
글로벌 패키징 솔루션
Thermo Fisher Scientific은 30년 이상의 글로벌 임상 패키징 및 라벨링 전문성을 바탕으로 워크플로우를 간소화하고 속도를 높일 수 있는 유연한 임상 서비스를 제공합니다.
Fact sheet
바이러스 벡터 서비스 인허가 지원 서비스
Patheon은 바이러스 벡터 서비스 20년 이상의 경험을 기반으로 세포 유전자 치료제를 위한 규제 컨설팅 서비스를 제공합니다. 당사 인허가 지원 서비스에 대해 자세히 알아보세요.

스마트 패키징 - 임상시험 결과에 대한 신뢰 획득하기
Patheon CDMO팀은 20년 이상의 스마트 패키징 경험을 갖추고 있습니다. 당사의 전문성을 확인하고 이를 활용하는 방법을 확인하세요.

Fact sheet
세포치료제 생산 서비스 살펴보기
세포 및 유전자 치료 시장은 가속화된 시장 승인 기회, 역대 최고의 투자, 견고한 치료 파이프라인, 긍정적인 임상 결과를 경험하고 있으며, 이에 따라 제조 기술의 속도, 규제 노하우, 혁신의 필요성이 커지고 있습니다.

Fact sheet
바이러스 벡터 개발 및 제조 서비스
Thermo Fisher Scientific은 바이러스 벡터 제품의 개발 및 생산에 있어 20년 이상의 경험을 자랑합니다. 선도적인 CDMO로서, 당사는 공정 및 분석 개발, 공정 밸리데이션, 임상 및 상업용 제조, 공정 중/출하 시험, fill-finish 서비스를 모두 포함하는 end-to-end 바이러스 벡터 서비스를 제공합니다.
Fact sheet
Direct-to-toxicology - 바이러스벡터 독성시험 서비스
독성연구는 IND 전 규제 요건을 뒷받침하고 약물의 위험성-유익성 비율을 평가하는 데 중요합니다. 당사의 바이러스벡터 direct-to-toxicology 프로그램은 아데노 연관 바이러스(AAV) 및 렌티바이러스(LV) 생산 공정을 통해 독성연구 물질로의 경로를 신속하게 처리하여, 이 과정을 최소 6개월 내에 완료합니다.

eBook
임상시험용 패키징 - 최신 기술을 이용한 유연하고 빠른 서비스
임상시험용 패키징은 약물에 따라 방식이 다양할 수 있습니다. 당사는 다수의 임상시험 지원을 통해 축적한 경험을 통해 의뢰자의 즉각적이고 장기적인 요구를 충족하는 솔루션을 제시합니다.
Blog post
순응도 및 정확성: 스마트 패키징으로 임상시험에서 품질 필수 요건 개선하기
Thermo Fisher Scientific의 약물 순응도 및 바이오마커 책임자로부터 스마트 패키징의 데이터 품질 영향과 이를 임상시험에 통합하는 방법에 대해 확인하세요.
15 minute read
Blog post
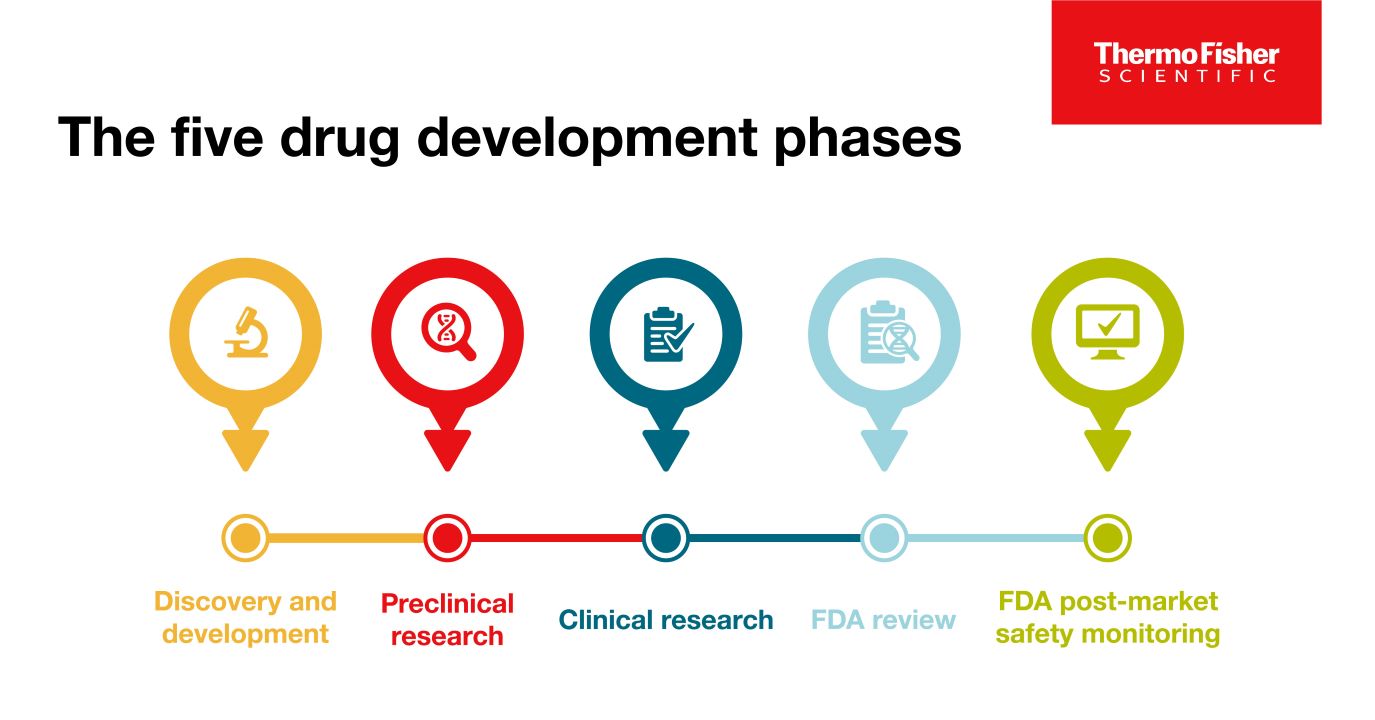
약물 개발의 5단계
성공적인 상업화를 위해서, 모든 약물은 1) 약물 발견 및 개발 2) 전임상 연구 3) 임상 연구 4) FDA 검토 5) 안전성 모니터링의 5가지 특정 단계를 통과해야 합니다.
9 minute read
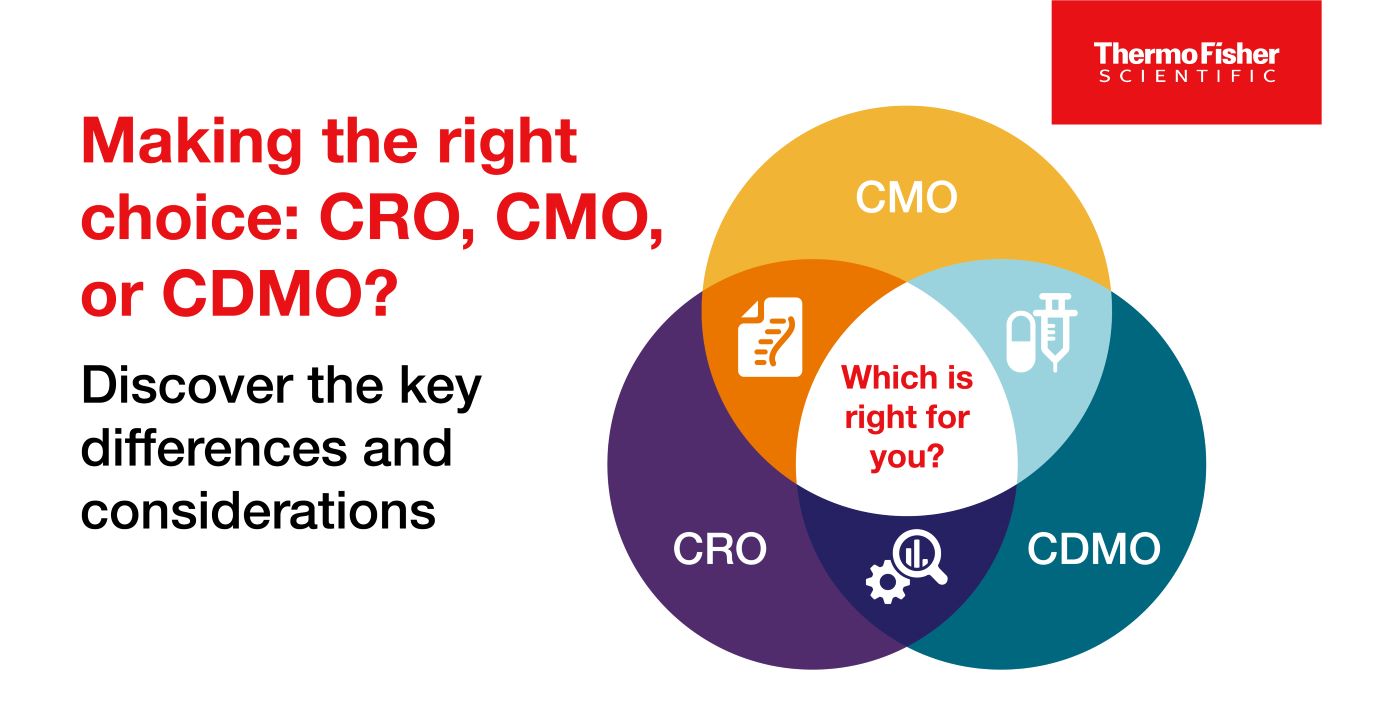
Blog post
CRO / CMO / CDMO 차이점
CRO, CMO, CDMO는 모두 약물 개발 및 제조와 관련된 서비스를 제공합니다. 자료에서는 이 세 조직과 서비스의 차이점을 설명합니다.
9 minute read

Blog post
세포 및 유전자 치료제 개발 - 환자 중심의 접근 방식
블로그 포스트를 통해 세포 유전자 치료제 개발의 환자 중심적 접근 방식에 대해 알아보세요.
8 minute read

Blog post
통합된 업무 프로세스를 통한 디지털 업무 환경 조성
안정성과 경험이 중요한 물리적 업무 환경과는 대조적으로, 디지털 업무 환경은 혁신과 연결성에 초점을 맞춥니다. 자료를 통해 디지털 업무 환경 조성에 대한 자세한 내용을 확인하세요.
4 minute read

Blog post
바이러스 벡터 상용화 – Part 1: 기술 이전 프로세스
기술 이전으로 바이러스 벡터 개발과 생산 규모를 확장하고, 백신 및 유전자 치료제 상용화 속도를 높이는 방법을 확인하세요.
9 minute read

인포그래픽
세포치료제 제조 워크플로우
인포그래픽을 통해 플라스미드 생산부터 콜드체인 물류에 이르기까지, 유전자 변형 세포 치료제 워크플로우의 개요와 각 단계의 주요 고려사항에 대해 확인하세요.

인포그래픽
mRNA 제조 워크플로우
인포그래픽에서는 mRNA 제조 공정에서 상호 연계된 각 단계를 탐색하고 Thermo Fisher Scientific의 유연한 접근법이 어떻게 귀사의 mRNA 치료제를 임상에 더 빨리 공급할 수 있는지 설명합니다.

인포그래픽
임상시험 성공을 위한 콜드체인 서비스
임상시험 요구를 충족하기에 충분한 콜드체인 역량을 보유하고 계신가요? 인포그래픽을 통해 임상시험을 성공으로 이끌기 위한 콜드체인 서비스 역량에 대해 알아보세요.
인포그래픽
바이오의약품 상업화 - 위험을 줄이고 결과를 최적화하기 위한 전략 이해하기
실험실에서 완제의약품의 최종 생산까지, 약물 생산에는 여러 단계의 공정이 발생합니다. 각 단계마다 서로 다른 전문가들이 개입하므로 다양한 작업이 동시에 진행되는 프로그램을 원활하게 진행하려면 계획 및 검증된 실행 접근법이 필요합니다.

인포그래픽
생체시료 저장(Biorepository): 자체구축 또는 아웃소싱 여부를 결정하기 위한 10가지 고려사항
온도에 민감한 생체시료를 사내에서 관리해야 할 지, 아웃소싱해야 할 지 결정하셔야 하나요? 자료를 통해 의사결정 시 고려해야 하는 10가지 요인을 확인하세요.
Whitepaper
Navigating the Adoption of Continuous Manufacturing Amid Unprecedented Global Challenges
연속 생산은 혁신적인 솔루션으로 배치 생산과 비교해 유연성을 자랑하며 스케일업 비용을 최대 1.65배까지 절감합니다.

Webinar
중국에서 임상 시 대조약 소싱에 대한 도전과 주요 고려사항
대조약 소싱은 임상시험에 있어 매우 중요한 요소이며 해외 임상 시에는 소싱 과정이 매우 복잡해집니다. 중국에서의 임상 시 대조약 소싱의 도전을 극복하기 위한 방법을 알아보세요.
Webinar
세포 치료의 환자 여정을 최적화하기 위한 CRO/CDMO 파트너십
통합 CRO/CDMO 파트너와 협업하는 것이 어떻게 업계의 도전과제를 완화하고 개발부터 생산까지 가속화된 경로를 제공하는 지 논의합니다. 또한 통합된 팀과 인프라에서 얻을 수 있는 혜택도 설명합니다.

Fact sheet
cGMP 생산을 위한 PoC 연구 지원
당사의 중개연구 서비스는 첨단 치료제의 개발부터 생산까지 모든 단계를 지원하여 관련 물질을 생성하고 개발에서 임상 생산 단계까지 원활한 전환을 보장합니다.

Webinar
유전자 치료제 개발 - 통합 접근 방식이 주는 혜택
유전자 치료제를 위한 바이러스 벡터의 개발과 상업 생산 과정에서 여러 도전 과제를 알아보고 프로젝트를 비용 효율적으로 성공시킬 수 있는 방향을 제시합니다. 통합적인 개발/생산 접근 방식을 활용하면 의사 결정을 간소화하고 자원을 효율적으로 활용함으로써 맞춤형 솔루션 이상의 혜택을 얻을 수 있습니다.

Whitepaper
품질에 대한 종합적인 이해를 통한 CDMO 파트너십 개선하기
CDMO의 품질을 판단하기 위한 주요 지표와 고객-CDMO의 파트너십을 지속적으로 개선하고 협력을 강화하여 궁극적으로 신뢰 관계를 구축하기 위한 도구와 모범 사례를 설명합니다.

Webinar
차세대 분석을 활용한 바이러스 벡터 생산
당사 분석 전문가가 바이러스 벡터 생산 워크플로우에서 분석법의 역할에 대해 설명하고, 정확도, 워크플로우의 복잡성, 처리량 제한과 같은 문제점에 대해서 논의합니다. 또한 사례 연구를 통해 혁신적인 방법론이 분석 품질과 재현성을 어떻게 개선할 수 있는 지 설명합니다.

Presentation
Preparing viral vector productions for commercialization
유전자 치료 백터가 급속하게 상용화 단계에 접어들면서 상용화 대비의 중요성이 커졌습니다. 웨비나를 시청하고 바이러스 벡터 상용화에 대비하는 당사의 역량과 접근방식에 대해 알아보세요.

Fact sheet
Early Development Solutions
의약품의 초기 단계 개발에서는 기업 규모와 관계없이 모두가 빠르고 비용 효율적이며 과학적 근거가 충분한 통찰을 얻는 것이 중요합니다...

Event
Tradeshow
AusBiotech 2023 — Brisbane, Australia
Visit booth #24-25 at AusBiotech 2023 to learn how our end-to-end CDMO services and solutions can support your unique drug development and manufacturing journey.

Event
Tradeshow
Meeting on the Mesa
Join us at the Cell & Gene Meeting on the Mesa, where our CDMO experts will be available to discuss how our expertise, capacity, and global network can address your project’s unique needs.

Blog post
Keeping your information safe: IP and data protections in China and across the globe
Experts at Thermo Fisher discuss rigorous contractual, project team, and data protections that are in place to keep customers’ intellectual property and confidential information secure.
12 minute read

Blog post
Manufacturing in China for China: Navigating the regulatory landscape
Experts at Thermo Fisher provide insight into the registration and approval pathway for developing and marketing biologic therapies in China for the domestic market.
12 minute read

Bioplus Interphex Korea 2023
Thermo Fisher Scientific이 7월 12일~14일 코엑스에서 개최되는 바이오플러스-인터펙스 코리아에 참가합니다.

Bioplus Interphex Korea 2022
Thermo Fisher Scientific이 BIX 2022에 참가합니다.
일시: 2022년 8월 3일 ~ 5일
장소: 코엑스 Hall A
부스번호: E10 Thermo Fisher Scientific

Expert
Matthew Jones, PhD
Senior Manager, Crystallization
Scientific Expertise:
- Crystallization process development, small molecules and biological macromolecules
- Particle engineering
- Downstream processes (solid-liquid separation and drying)
- Solid state chemistry and solid state analysis
Focus Area
API (small molecule)
Credentials
Habilitation (higher doctorate) in chemical engineering from MartinLuther-University Halle-Wittenberg, Halle (Saale), Germany
Doctor of Philosophy from the University of London, University College, U.K.

Expert
Michael Cruskie, PhD
Sr. Director and General Manager, API (Small Molecule)
Scientific Expertise:
- Product development
- Small molecule APIs
- Strategy & process development
- Process improvement/optimization
- Operations and manufacturing
- Technology transfer
- Scale-up
- Supply chain
- Outsourcing
Focus Area
API (small molecule)
Credentials
Doctor of Philosophy in organic chemistry from the University of Florida
Bachelor’s degree in chemistry and mathematics from St. Lawrence University
Webinar
Gateway to China: Developing Biologics for the fastest-growing patient population on Earth
중국은 미국에 이어 세계에서 두 번째로 큰 제약 시장으로, 2021년 약 12%의 세계 시장 점유율을 차지합니다. 인구 고령화와 새로운 적응증의 확대로 중국은 모든 바이오 제약 회사에게 매력적인 시장으로 인식되고 있습니다.

Webinar
Technology transfers: Best practices for optimizing success and mitigating risk
글로벌 네트워크를 보유한 CDMO 파트너와 협업하는 기업은 생산시설 간 기술이전을 통해 다양한 전략적 이점을 얻을 수 있습니다.

Webinar
The future of decentralized clinical trials in an evolving EU regulatory landscape
코로나19 팬데믹을 계기로 현장 중심 업무였던 임상시험을 완전 분산형 또는 하이브리드형으로 조정 가능함이 입증되었고 전세계적으로 분산형 시험이 임상연구를 가속화하는 안전하고 효과적인 대안으로서 받아들여지게 되었습니다.

Whitepaper
기술이전, 위험은 축소하고 혜택은 확대하는 방법
2020년 당사는 181건의 기술 이전을 성공적으로 마무리함으로써 고객의 공급 확보, 유통 개선, 프로그램 비용과 위험 절감을 지원했습니다.
Whitepaper
mRNA vaccine development: Key insights for planning, workflow, and supply chain success
mRNA 코로나19 백신 개발에서 얻은 주요 인사이트와 향후 mRNA 백신 제조 및 치료법의 발전을 가속화시키기 위하여 이를 응용하는 방법을 확인하실 수 있습니다…

Fact Sheet
cGMP 세포 치료제 제조 서비스
당사는 세포 유전자 치료제의 생산 속도를 가속할 수 있는 경험과 역량을 기반으로 개발부터 유통까지 통합 서비스를 제공합니다...

Blog post
Viral vector commercialization – Part 3: Specialized regulatory support
Find detailed regulatory considerations when preparing viral vectors for commercialization and best practices to address them.
7 minute read

Blog post
Ensuring Quality Consistency at Hangzhou Site
Find Q&A insights from experts at Thermo Fisher on the quality systems framework that guides quality implementation at our new Hangzhou site.
12 minute read

Blog post
Patheon Translational Services Advance Cell and Gene Therapies from Research to Clinical Trials
Learn how our translational research services, housed in our San Diego facility, can take cell and gene therapies from preclinical to clinical.
6 minute read

Blog post
Top tips for providing the right amount of detail in first-in-human common technical documents
In the early-development stage, little may be known about a drug’s characteristics. What’s more, drug processes and formulations frequently evolve as more information emerges following testing and trials. Learn more.
5 minute read

Blog post
Gaining a deeper understanding of your API
As active pharmaceutical ingredients (APIs) become increasingly complex, they pose potential formulation problems that can extend timelines and explode budgets. Read this blog to learn more.
5 minute read
Blog post
EU and US regulations: What’s coming for cell and gene therapies?
Cell and gene therapy (CGT) developers today face an added challenge in their quest to bring a product through clinical trials and to the market. Read this blog to learn more.
7 minute read

Blog post
Trends in mRNA therapeutics: Pandemic learnings for a pathway to success
The rapid advancement of the Pfizer-BioNTech and Moderna messenger RNA–based COVID-19 vaccines from lab to clinic—with development taking less than one year—has validated the...
5 minute read
Blog post
Choosing a CDMO for mRNA success: Five CDMO characteristics needed
The promise of mRNA technologies has been clearly demonstrated during the COVID-19 pandemic, with vaccines reaching the market in record time. The vital role...
7 minute read

Blog post
Moving from vials to prefilled syringes for vaccines: Three key success factors
As pharmaceutical companies become more patient-centric and self-administration of injectable drugs continues to increase, the market for drug products in prefilled syringes is forecast to grow, reaching $9.53 billion by 2026.
4 minute read

Blog post
Choosing a CDMO Who is a True Bioproduction Expert
With the number of CDMO’s rising in the biologics manufacturing industry, it can be challenging for new and emerging biopharmaceutical companies to determine which CDMO is right for them.
8 minute read

Blog post
Introducing an Expanded Packaging Service for Specialty Products
Developing a specialty drug for a complex or rare disease is an achievement worthy of celebration.
2 minute read

Blog post
Top Tips on How to Manage Clinical Label Translation and Regulatory Requirements
Wouldn’t it be nice to find a way to stay on top of label translation and regulatory requirements for every country included in your clinical trial?
2 minute read

Blog post
Is Your Supply Chain Bulletproof?
Before you respond, think hurricanes, Nor’easters and tsunamis. Earthquakes, typhoons and volcanic eruptions. Civil unrest and war. Terrorism. A pandemic virus.
2 minute read

Blog post
Myths & Facts about Ancillaries
It’s a fact that ancillary supplies are frequently perceived as less important than study drug.
2 minute read

Blog post
The Four Stages of Equipment Qualification
As discussed in my previous blog, qualification is the process of establishing documented evidence that a specific equipment, facility or system are fit and ready for their intended use.
3 minute read

Blog post
Navigating Cell & Gene Therapy Regulations: How Does Your CDMO Match Up?
Whether you are a large or new and emerging biotech company, many companies find themselves lacking the internal resources and/or expertise to properly support regulatory submissions.
7 minute read

Blog post
Prefilled Syringes: Three Pain Points You May Not Have Considered
As pharmaceutical companies look to become more patient-centric, certain drug products come to the forefront to support that effort.
5 minute read

Blog post
Work Smarter, Not Harder: Accelerating Your Biologics Development and Commercialization
Over the past decade, the biologics industry has seen double digit growth and an overall increase of market share.
5 minute read

Blog post
How Decentralized Clinical Trials Enhance Patient-Centricity in the Age of COVID-19
As COVID-19 continues to change how we do business in the biopharmaceutical industry, it’s important to not lose sight of why we do what we do: improving and saving patient lives.
5 minute read

Blog post
A Day in the Life of a Viral Vector Partner
When it comes to a viral vector Contract and Development Manufacturing Organization (CDMO), what sort of qualities should they possess?
9 minute read

Blog post
Continuous Manufacturing: An Efficient Way to Produce OSD Drugs
When Henry Ford revolutionized manufacturing practices back in 1913 in Highland Park, MI, his main goal was simple—to make the best possible product in the most efficient and cost-effective manner. Ford’s focus on bettering the “flow” of manufacturing to enable workers/technology to work smarter and reduce waste of raw materials, changed manufacturing principles forever.
6 minute read

Blog post
COVID-19’s Silver Lining: Accelerated Vaccine Development
Vaccine development is a lengthy process—it is expensive, attrition is high, and to get a licensed vaccine to everyone, it takes multiple candidate iterations. Vaccine development for pandemics and epidemics is risky, and due to the novel nature of viruses, certain unknown factors can derail a vaccine program.
6 minute read

Blog post
Simplifying Your API's Development Journey
The primary way new drugs are developed is changing. Historically, pharma companies—particularly large pharma companies—have developed APIs in-house.
7 minute read

Blog post
Considerations and roadblocks that stifle orphan drug development
According to the US Food and Drug Administration (FDA), “2020 was a record-breaking year in terms of the number of orphan drug designation and rare pediatric disease designation requests submitted to the Office of Orphan Products Development.”
6 minute read

Blog post
Break Through the OTC Noise
It’s no secret—consumers have a vast range of OTC options at their favorite in-store or online retailer. Everything from tablets and capsules, to syrups—consumers have more options than ever in the OTC jungle.
8 minute read

Blog post
Taking Your API to the Next Level: Three Steps to Consider Before Outsourcing
With outsourcing API development becoming more common, we see a rise of multiple competing Contract Development Manufacturing Organizations (CDMOs) as potential development partners.
10 minute read

Blog post
Is it Time to Start Thinking about Packaging?
If this headline caught your eye, it may be because you’ve received promising pre-clinical results (Congratulations!) and you’re starting to think about planning your next steps.
2 minute read

Blog post
Time to embrace electronic labels? Potential labeling solutions under the new EU Clinical Trial Regulation
The new EU Clinical Trial Regulation (CTR) is intended to simplify clinical trial administration and create a more welcoming climate for pharmaceutical companies that operate in Europe.
5 minute read

Blog post
Navigating the Complexities of Process Performance Qualification
Method qualification is monumentally important before process performance qualification (PPQ). This early assessment of your method’s performance characteristics is critical as it pertains to method validation and its parameters such as precision, accuracy, and linearity.
5 minute read

Whitepaper
Accelerating Biopharmaceutical Development with Strategic CDMO Partnerships
불확실성이 증가하는 변혁의 시기에 바이오 의약품 개발을 성공으로 이끌기 위해서는 변화의 원동력이 되는 과학, 기술, 시장의 변화를 이해하고 이에 맞는 전략을 도입해야 합니다.

Whitepaper
What You Need to Know About Process Characterization and Validation For Biologic Processes
지난 20년 간 바이오의약품 업계 성장의 주요 요인은 단클론 항체(mAb)를 이용한 지속적인 혁신이었으며, 현재 mAb는 전체 바이오 치료제의 50% 이상을 차지하고 있습니다.

Whitepaper
Top five risks facing your small biopharma clients
바이오 제약 기업은 한정된 예산과 시간이라는 리스크가 높은 개발 환경에 놓여 있습니다. 압박이 심한 환경에서 기업은 향후 일정을 지연시키거나 또는 개발 중단을 일으킬 수 있는 중요한 요소를 간과하는 경향이 있습니다...

Whitepaper
Telltale signs you’re with the wrong CDMO
신약 개발 후기 단계에서는 상업화가 추진되면서 프로젝트에 많은 변경이 발생하기 마련입니다. 이런 중요한 시기에 기업은 벤더를 평가하여 상업화를 위한 옵션을 분석하게 됩니다. 상업화를 위한 스케일업 단계에서 당신의 CDMO가 실제로 힘이...

Whitepaper
Quality by Design: A Holistic Approach to Drug Development
바이오 제약 산업의 발전이 계속됨에 따라, QbD (Quality by Design)는 약물 개발 및 생산에 대한 포괄적이고 선제적인 접근 방식으로 핵심 프로세스를 혁신하고 있습니다....

Whitepaper
Rise in Targeted Therapies Drives Need for Small-Volume Manufacturing
바이오 의약품 시장은 지난 15년간 꾸준히 두 자릿수 성장세를 보였으며 현재는 전체 FDA 신약 승인 건의 4분의 1 이상을 차지하고 있습니다. Evaluate Pharma의 희귀의약품 프로젝트에 관한 2017년 보고서에 따르면...

Whitepaper
Six API Challenges That Could Be Slowing Your Development and How to Avoid Them
시장에 진입하려는 신약 앞에 놓인 문제는 다양합니다. 그러나 많은 문제는 자초되는 것이고 특히 이는 저분자 개발에서 두드러집니다. 매우 강력한 API를 제외하고 저분자 약물은 공정...

Whitepaper
Novel Uses for Oral Solid Doses Driving Lifecycle Management Strategies
현재 제약업계 파이프라인에 잠재적인 블록버스터 의약품이 거의 없는 상황에서 제약사들은 경구제(OSD) 분야에서 환자의 요구를 충족시키고 매출을 늘릴 수 있는 다른 옵션을 찾고 있습니다...

Whitepaper
Technology Transfers: Reaping Rewards, Reducing Risks
2016년 Patheon은 111건의 기술 이전을 성공적으로 마무리함으로써 고객의 공급 확보, 유통 개선, 프로그램 비용과 위험 절감을 지원했습니다. 기술 이전 과정에서 고객사는 Patheon의 전문지식, 안정적인 공정, 표준화된 작업 절차와 장비를 활용...

Whitepaper
Multiplexing: Managing risk with proven, single use solutions
기업은 신약 출시에 막대한 자본을 투자하면서 마찬가지로 큰 위험을 감수하게 됩니다. 일반적으로 신약의 대규모 생산 역량을 마련하는 데에는 3~4년이 소요되므로 임상 3상이 완료되기 전에 생산량을 결정해야 합니다...

Whitepaper
Is Your In-House Strategy Ready for the Uncertainties of Biologic Drug Development?
블록버스터 약물에 대한 과거 제약업계의 의존성은 소규모 환자 집단의 충족되지 않은 요구를 처리하는 약물 개발에 초점을 맞추는 것으로 진화했습니다.

Whitepaper
Strategies for API Solubility and Bioavailability Enhancement
오늘날의 신약 개발 환경은 10년 전과 비교해도 확연히 달라졌습니다. 기술의 혁신은 환자 치료에 있어 새로운 가능성을 불러 일으켰습니다...

Whitepaper
In-House Versus Outsource: A Decision-Making Guide
바이오 의약품 시장은 블록버스터 의약품 생산에서 미충족 수요의 치료제를 연구하는 틈새시장으로 이동하고 있습니다. 이로 인해 경쟁이 심화되고 개발 일정이 촉박해지며 역량 부족의 문제가 대두되는 등 후보물질의 성공적인 개발을 방해하는 여러 위험 요소가 제기되고 있습니다...

Whitepaper
Getting to First-in-Human Clinical Trials: A Make-or-Break Milestone for Small Biopharmas
의약품의 신속한 출시에 대한 기대가 커지면서 '더욱 빠르고 효과적인 의약품 개발'은 바이오 제약 기업의 목표가 되었습니다. 최초로 출시된 의약품이 높은 시장 점유율을 확보할 수 있다는 사실은 모두가 잘 알고 있습니다...

Whitepaper
Full throttle for vaccine filling
팬데믹 기간동안 업계는 비즈니스 지속성, 신속한 상업화, 공급망 효율성을 모두 달성할 수 있도록 급변하는 환경에 빠르게 적응해야...

Whitepaper
Fixed-dose combination drugs: Innovative formulation and development strategies for bringing best-in-class products to market
고정용량복합제(FDC)는 고혈압, 당뇨, 암, 결핵, 천식, COPD를 비롯한 다양한 질병과 질환을 치료할 때 단일요법의 중요한 대안이 되었으며, 더 많은 질병을 치료할 가능성이 있습니다...

Whitepaper
Rising to the rare disease challenge: Key considerations in large-molecule orphan drug development
희귀의약품은 소규모 제조, 한정된 시간/데이터, 적은 시료 규모, 비용 때문에 신약 개발 분야에서도 특히 더 어려운 도전 과제입니다. 다음을 통해 이런 어려움을 극복하는 방법에 대해 확인하세요....

Whitepaper
Factoring the “what ifs” into supply forecasting: Why building a durable supply chain around a protocol is critical
임상 공급은 그간 임상 계획 과정의 핵심 요소가 아닌 실행 단계의 일부로 간주되어 왔습니다. 현재는 신제품을 시장에 빨리 출시하려는 바이오 업계의 경쟁으로 인해 공급 예측이 전략적이고 매우 복잡한 성공 요인으로 인식되고 있습니다...

Whitepaper
Continuous manufacturing and late-phase strategy: The time is now
수록 많은 기업이 다음과 같은 목표를 갖고 연속 생산 활용에 따른 장점과 장기적 비즈니스 영향을 탐구하고 있습니다...

Whitepaper
Considerations for your first clinical trial
수년간의 연구와 여러 차례의 펀딩 끝에 마침내 첫 임상을 시작할 준비가 되었습니다. 의약품 개발은 경쟁이 치열하며 따라서 진행 속도가 절대적으로 중요합니다. 전체 개발 단계를 통과하지 못하는 후보물질이 상당수라는 점을 고려하면...

Whitepaper
Challenges and Practical Solutions for Switching to Prefilled Syringes for Injectables
개발 및 임상 단계에서 바이알 등 다른 제품 형태를 사용한 후 상업용 의약품 출시에 앞서 사전 충전형 주사기로 전환하는 경우가 있습니다. 제형 전환은 새로운 투자를 요구할 뿐 아니라 환자와 이해당사자들에게 개선된 결과를 가져오므로...

Whitepaper
Biologic drug products: A 5-point strategy for building a robust CMC dossier
후보물질의 성공적인 개발과 생산을 위해서는 개발 초기부터 CMC의 규제 지침을 이해하고 자료를 철저히 준비하는 것이 매우 중요합니다...

Whitepaper
Patient-centric oral solid dose formulation: Improving access and value across the product lifecycle
환자들은 자신의 건강을 관리하는 데 있어 중추적이고 선제적인 역할을 수행하고 있으며, 이는 환자를 좀 더 중심에 두는 약물에 대한 수요를 촉진하고 있습니다…

Whitepaper
Analytical Considerations For Biopharmaceuticals During Commercialization
의약품의 품질과 안전성을 보장하려면 적격성평가(PPQ) 전에 분석 방법을 검증해야 합니다. 또한 의약품이 규제 기관의 품질 기준을 충족한다는 것을 입증하기 위해서는 높은 수준의 재현성과 정확도를 갖춘 강력한 분석 방법이 필요...

Whitepaper
Sterile formulation strategies to shorten timelines for first-in-human studies
최근 몇 년 동안 희귀의약품, 빠른 임상 종료점을 가진 적응증에 대한 임상 후보물질이 늘어나면서, 후기 임상 단계로의 경로가 짧아지고 가교 연구를 수행할 시간이 줄어들었습니다. 따라서 보다 확장 가능한 제형 개발에 대한 필요성이 대두되고 있습니다....

Whitepaper
Addressing the complexity of process validation for cell and gene therapy products
세포 유전자 치료제의 복잡성으로 인해 치료제의 개발과 상업 생산 주기 전반에 걸쳐 공정에 대한 깊은 이해가 요구되고 있습니다. 규제 당국은 세포 유전자 치료제에 있어서 잠재적인 변동성의 원인을 식별하고...

Whitepaper
Understanding the CMC regulatory landscape for cell and gene therapy products
규제 당국에 대응할 준비가 되어 있지 않으면 의약품 출시는 지연될 수밖에 없습니다. 대기업이든 바이오 벤처든, 기업 규모에 관계없이 많은 기업에서 서류 준비 과정 중에...

Whitepaper
Impact of a pandemic outbreak on vaccine development approach
백신을 개발 과정에는 많은 장애물이 존재합니다. 그러나 팬데믹 기간 동안의 백신 개발에 있어서는 기존의 제조 방식으로는 해결할 수 없는 고유의 복잡한 문제가 존재합니다. 지난 10년간 H1N1 인플루엔자, 사스-코로나바이러스(Sars-Cov), 에볼라, 메르스의 발생은...

Whitepaper
How can you avoid the fallout from incompatibility between your API and its formulation?
약물 개발 과정에서 완제의약품(예: 정제)의 제형을 설계할 때 활성 제약 성분(API 또는 원료의약품)의 물리적 및 화학적 특성 모두에 주의를 기울여야 합니다...

Whitepaper
5,000L single-use bioreactors: The next generation in biologics manufacturing
글로벌 바이오 의약품 시장과 연구 발전의 지속적인 확대에 따른 치료제 수요 증가는 바이오 의약품과 바이오시밀러 포트폴리오 확대를 가져왔습니다...

Whitepaper
The Economic Advantage of Single-Source Drug Development and Manufacturing
터프트 신약 개발 연구 센터는 최근 단일 벤더 생산과 복수 벤더 생산의 경제성 평가라는 제목의 연구를 통해 다중 및 단일 소스 CDMO 모델 간 일정과 개발 경제성을 비교했습니다...

Infographic
CDMO Checklist to Launch Your Molecule Globally
글로벌 시장에 의약품을 출시하기 위해서는 CDMO 가 속도, 안정성, 공급량 확보의 조건을 갖추고 글로벌 규제 환경에 잘 대처할 수 있는 지 확인해야 합니다. 자료에서는 CDMO 평가를 위한 옵션을 제공합니다.

Infographic
A practical guide to writing robust chemistry, manufacturing, and controls dossier modules to support first-in-human trials
인간 대상 첫 임상시험의 확고한 근거가 되는 CMC 작성을 위한 실용적인 가이드를 소개합니다.

Infographic
Advances in viral vector manufacturing
모든 상황에 맞는 만능 바이러스 발현 시스템은 존재하지 않습니다. 항상 트레이드오프 관계에 있으며 매번 상황에 따라 선택해야 합니다. 인포그래픽을 다운로드 하여, 경로를 결정하기 전 선택지를 고려해보세요.

Infographic
The unrealized value of a combination drugs strategy
임상적 장점으로 고정 용량 복합제(FDC) 인기가 높아지고 있습니다. FDC는 비즈니스 관점에서도 기업에 혜택을 제공하기도 합니다. 인포그래픽을 다운로드하여 자세한 내용을 확인하세요.

Infographic
Regulatory pathways for CGT and ATMP products
세포 유전자 치료제(CGT)는 현재 세계에서 가장 빠르게 성장하는 치료 분야 중 하나입니다. 이러한 치료법은 환자를 평생 치료하는 아니라 완치를 목적으로 합니다. 절차를 간소화하려는 노력의 결과로 CGT 제품의 개발 일정은 기존 대비 단축되었으며, 이에 따라 제약사는 특수한 도전과 기회를 얻게 되었습니다. CGT 업계가 성숙함에 따라 지침과 규제 표준이 지속적으로 엄격해지고 진화하고 있으므로 기업은 의약품의 성공을 위해 규제 당국과 밀접하게 협력해야 합니다.

i
A Tale of Two- CDMOS Which Choice Will Your Biopharma Make
바이오의약품 개발 시 마주하는 도전과제가 늘어가면서 기업에게 CDMO의 역할은 더욱 중요해지고 있습니다.

Infographic
Real-time Track and Trace platform for clinical trial shipments
Thermo Fisher Scientific의 이력추적 솔루션은 사내외 임상시험 이해당사자들에게 배송중인 모든 임상시험 재료에 대한 주문형 배송 업데이트를 제공합니다...

Infographic
Made with proof & purpose
최신 인포그래픽을 통해 고객의 후보물질을 성공으로 이끄는 Thermo Fisher Scientific의 역량을 확인하세요...

Infographic
Direct to Patient Pharmacy Services
임상시험 품질을 저해하지 않고 시장에 출시하는 속도를 확보하기 위해서는 임상시험을 가속화하는 것이 가장 중요한 성공 요인입니다. 그러나 기존의 임상시험 모델은 이러한 목표에 적합하지 않습니다...

Infographic
A Tale of Two Molecules
용해도가 낮은 의약품을 위한 최적의 솔루션은 무엇일까요? 두 후보물질은 모두 동일한 증상을 치료하기 위해 고안되었으며, 모두 용해도 문제가...

Infographic
Depot to Patient Service Overview
환자에게 직접 의약품을 배송하는 사례가 점차 확대되고 있습니다. 이 서비스에는 임상시험 사이트에서 환자에게, 디포(Depot)에서 환자에게, 그리고 약국에서 환자에게 전달하는 옵션...

Infographic
Four special fill/finish considerations for vaccine production
백신 생산 준비가 완료된 후에는 처리 및 충전/완제 공정에 관해 고려해야 할 중요한 문제가 있습니다. 올바른 선택은 백신의 안정성과 순도를 보장할 뿐만 아니라 불필요한 낭비와 비용도 줄입니다...

Infographic
Top strategic tips for filing a successful IND with speed and efficiency
IND, IMPD 성공적인 신청을 위한 8가지 팁을 소개합니다...

Infographic
Decision point: Build vs. buy continuous manufacturing
연속 생산 공정은 비용 절감의 유익성으로 인해 업계 내에서 빠르게 확산 및 채택되고 있습니다…

Infographic
Small Pharma, Big Opportunity: Should CDMO Partner Size Influence Selection for your Large Molecule Project?
초기 개발 과정에서 리소스 제약과 공정 개발 문제로 인해 기업은 위탁 개발, 생산을 찾는 경우가 많습니다. 특히 신생 바이오기업은 CDMO 규모를 결정하는 데 있어 어려운 결정을 내리는 경우가 많습니다...

Infographic
10 Reasons Formulation Complexity is on the Rise in Steriles
초기 개발 기간에는 제제의 복잡성 등 의약품 연구를 다음 단계로 진행하는 데 방해가 되는 다양한 도전과제가 존재합니다. 다양한 요인으로 인해 무균 주사제 개발의 복잡성은 점점 더 증가하고 있습니다. 특히 이는 업계 전체가 다음 이슈에 중점을 두고 발전하기 때문에 발생하는...

Webinar
Benefits of 5KL Bioreactor When Outsourcing Late-phase Biologics Drug Substance Manufacturing
현재 업스트림 바이오 공정에서는 두 가지 중요 요소가 주목을 받고 있습니다. 첫째는 바이오리액터의 용량, 특히 혼합과 물질 전달 성능 개선에 대한 과제를 제기하는 강화된 세포 배양 공정 입니다...

Webinar
Preparing for an evolving regulatory landscape to successfully commercialize cell and gene therapies
세포 및 유전자 치료는 임상시험을 통해 진보하며 빠른 속도로 상업화로 나아가고 있습니다. 그 결과, 규제기관은 개발 속도를 뒷받침하기 위해 빠른 속도로 규제를 변경하여 안내하고 있습니다...

Webinar
Accelerating Cell Culture Development through Practical Application of Innovation Technologies
상업화 니즈에 있어서의 변경뿐 아니라 바이오의약품 파이프라인의 변경에 따라서도 사용하는 기술과 세포주 개발 워크플로우가 변경될 수 있습니다. 혁신적인 기술을 통해 보다 빠르고, 효율적이고, 규제에 대비한...

webinar
FIH (First-in-Human) strategy for reducing the development period of oral and injection drugs for clinical use
기업은 초기 개발 시 일정 지연을 자주 겪곤 합니다. 적합한 솔루션을 활용하면 속도, 품질, 위험완화 사이의 균형을 유지하면서 개발 일정을 단축할 수 있습니다.

Webinar
Practical Implementation of Innovative and Scalable Technologies to Accelerate Biologics Development and Commercialization: A CDMO Perspective
웨비나를 시청하시고 혁신적인 신기술과 최고의 생산 역량을 토대로 바이오의약품 개발 시 발생하는 문제를 해결하는 방법을 확인하세요.

Webinar
Leveraging Innovative Technologies, Best Practices and Strategies to Accelerate Biologics Development and Commercialization
웨비나에서는 다중 속성 방식,고수율 세포주 및 자동화 시스템을 활용한 현대화된 세포주 개발 워크플로우, 차세대 정제 레진과 같은 혁신적인 기술이 바이오 의약품의 개발과 상업화를 가속화하는 데 어떤 도움을 줄 수 있는 지 설명합니다.

Webinar
Critical quality attributes (CQAs) of plasmids for cell gene therapy development
플라스미드 DNA는 세포/유전자 치료제의 원료 물질입니다.

Webinar
Biopharmaceutical development opportunities and benefits in Australia
연구개발 비용에 대한 세제혜택과 호주의 신속한 임상시험 인허가 제도로 신약개발을 위한 해외 임상시험 국가로 호주를 선택하는 바이오 기업들이 증가하고 있습니다.

Webinar
Vaccine Development: Strategic Approach & Response During Pandemics
지난 10년간 H1N1 인플루엔자, 사스-코로나바이러스, 에볼라, 메르스의 발생은 기존의 백신 개발 방법에 대한 몇 가지 논의를 불러 일으켰습니다. 사스-코로나바이러스-2의 확산이 심화되면서 과학자들은 제약사와 함께 품질이나 안전성, 효능에 영향을 미치지 않고...

Webinar
Transportation Solutions for Cell and Gene Therapy
세포 및 유전자 치료제 유통과 공급의 최우선과제는 의약품의 무결성을 유지하면서 목적지까지 정시 도착과 보관 온도 조건을 준수하는 것입니다. 세포 유전자 치료제는 운송 시 속도, 온도, 무결성을 유지해야...

Webinar
Stabilizing your supply chain in times of global volatility
완제의약품을 적시에 제조하여 환자에게 공급하려면 안정적인 공급망이 있어야 합니다. 그러나 글로벌 경제가 전쟁, 기후변화, 노동력 부족으로 불확실성이 증가하면...

Webinar
Simplify Your Supply Chain in the Race to Phase 1
임상 1상 목표를 달성하는 데 있어 공급망 단순화의 역할과 혜택에 대해 소개합니다. 또한 최근 개선된 Quick to Clinic...

Webinar
Regulatory uncertainties: Continuous manufacturing
연속 생산 공정을 광범위하게 채택하지 못하는 가장 큰 장벽 중 하나는 바로 불확실성입니다. FDA 승인 시기의 불확실성은 배치 생산에서 연속 생산으로 전환할 때, 혹은 연속 생산 공정을 이용하여 제품을 개발 및 출시할 때...

Webinar
QP release in the EU in 2022 and beyond: Your questions answered
QP(Qualified person) 감사에 대한 규정에 중대한 변경이 진행되는 가운데, 국제 임상시험 시장에서 성공적으로 길을 모색하고 임상시험용 의약품(IMP)의 시의적절한 공급을 보장하기 위해서…

Webinar
QP Release for drug products in Europe
복잡한 글로벌 임상시험을 진행하고 IMP를 제때 공급하려면 규제에 대한 깊은 이해가 필수적입니다. 유럽 이외 지역에 있는 제약사는 유럽의 GMP 요건, QP의 역할과 책임을 파악해야 합니다...

Webinar
Process Characterization: Ready for the FDA/EU ICH guidelines
공정 밸리데이션은 모든 상용화 제품의 요건이라는 것이 바이오의약품 규제당국의 공통적인 견해입니다. 이런 활동은 위험을 최소화하고 제품 Lifecycle 전반에 걸쳐 품질을 보증함으로써 환자를 보호합니다...

Webinar
Orphan Drugs: Balancing Financial Incentives & Complex Challenges
희귀의약품은 환자 수가 적은 것에 비해 개발과 출시 비용은 높습니다. 고위험 틈새 시장임에도 불구하고 희귀의약품은 신약 개발 시 받게 되는 금전적 인센티브와 혜택 때문에 시장 규모가 증가하는 추세에 있습니다...

Webinar
Navigating Decision Points to Fast-Track Commercialisation
의약품을 검증하고 출시하는 데 따르는 위험과 요건, 도전 과제를 파악하는 것은 바이오의약품 개발 프로젝트 성공의 필수 요소입니다. 자원의 제약, 전문적인 기술 수준의 부족 등 어려움을 겪고 있다면 특히 초기 개발 단계에서 주요 결정 사항을 탐색하는 것은...

Webinar
mRNA vaccines, trends, technologies and supply chain
이 웨비나에서는 코로나19 팬데믹이 백신 개발 접근법에 미친 영향에 대해 설명합니다. mRNA 백신 기술의 역할과 적용, 잠재력, 그리고 견고한 글로벌 백신 공급망 구축을 위해 해결해야 할 문제와...

Webinar
Manufacturing Innovation: The Case for Continuous Manufacturing
CDMO가 경구제의 연속 생산 공정을 제공함에 따라 이 제조 기술을 활용하고자 하는 기업은 과거와는 다른 비즈니스 케이스를 확보할 수 있게 되었습니다. 최근까지만 해도 기업이 상업 생산 공정에 연속 생산을 도입할 수 있는 유일한 방법은 초기에 상당한 자원을 투입하여 기술 역량을 개발하는 것이었습니다...

Webinar
Leveraging Infrastructure Investments and Innovation to Accelerate Biologics Development
바이오의약품과 재생의료 치료제의 파이프라인이 성장하고 새로운 바이오의약품 및 바이오시밀러 승인 속도가 빨라지면서, 큰 잠재력을 가진 후보물질을 성공적인 치료제로 전환할 수 있는 솔루션이...

Webinar
ISPE Virtual Inspections: Navigating the New Paradigm
Understanding the regulatory landscape for biologics is essential.

Webinar
How Adaptable Manufacturing Models are Paving a Steady Path into an Unpredictable Future
In a short 20 minutes, you can learn about manufacturing trends, adaptable manufacturing models, and take a tour of Pacira’s condominium manufacturing facility at Patheon’s Swindon location.
Webinar
Highly potent strategies from early development to commercialization
제약 R&D 분야는 과거 어느 때보다도 특수 의약품 개발에 집중하고 있으며 그로 인해 고효능 API가 늘어나고 있습니다. 이에 따라 지난 몇 년간 고효능 의약품 제조가 꾸준히 늘고 있으며 이런 동향은...

Webinar
Getting your global, small molecule CMC regulatory strategy right from the start
개발 업무에만 집중하다 보면 규제 요건을 종종 놓치게 되고, 이는 프로젝트 지연과 추가적인 비용을 야기할 수 있습니다. 따라서 개발 초기 단계에 CMC 전략을...

Webinar
Getting from R&D to IND – Pitfalls to avoid and how to succeed
IND까지의 개발 과정에는 언제나 어려움이 따릅니다. 개발 속도, 위험 완화, 미래의 가치 사이의 균형을 맞추는 것은 쉽지 않습니다. 향후 스케일업과 상용화를 위한 안정적인 기반을 확보하는 동시에 위험 완화와 일정 단축 사이의 균형을 찾아 후보물질에서 FiH 임상시험까지 진행...

Webinar
From Patient Adherence to Manufacturing Ease—Why Softgels Make Sense for Rx
연질캡슐은 처방약에서 많이 사용되는 제형은 아닙니다. 제약사는 처방약에 있어 정제와 캡슐을 주로 개발하는 경향이 있습니다. 그러나 연질캡슐은...

Webinar
Fixed dose combination drug development: Designing a lifecycle strategy with agility & speed
FDC와 약물 재창출은 의약품의 Lifecycle 관리 전략의 일환으로 일반적으로 채택되고 있습니다. 이러한 전략은 새로운 적응증에서의 부작용이나 임상 효능 평가를 줄일 수 있다는 장점이 있습니다. 다양한 이유로 심사가 보류된 의약품을 검증된 안전한 승인 의약품과 조합하면...

Webinar
FDA Accelerated Approval Pathways for Cell and Gene Therapy Products
세포 유전자 치료제 시장은 급변하고 있으며, 그에 따라 규제 가이드라인 또한 빠르게 변화하고 있습니다...

Webinar
Establishing a correctly characterized molecule in early drug development
고형 API의 물리적 특성은 주로 분리, 정제, 형태 제어에 이용되는 결정화 공정을 결정하고, 효과적인 제형 개발로 이어질 수 있게 합니다. 따라서 관련 고체상태 특성 및 고형-액상 평형에 대한 이해가 화학적 공정 및 제품 개발 모두에 핵심적…

Webinar
Smart packaging: The power of adherence data
각 환자의 투여로부터 수동적으로 수집된 개별 데이터 요인의 가치는 종종 가려져 있습니다. 이러한 데이터 요인을 시간에 걸쳐 종단적으로 보면, 개별 환자의 투여 행동에 대한 깊은 이해를 할 수 있게 되며…

Webinar
Essential up-front planning for your clinical trial
임상 공급망을 조기에 계획하면 위험과 비용의 효율적인 균형을 유지할 수 있습니다. 자원과 시간이 제한되어 있는 경우, 조기에 계획을 세우면 재고 부족과 연구 지연을 피하기 위한 위험 완화 전략을 포함해 주요 매개변수를 최적화하여 폐기를 최소화하고...

Webinar
Entering first-in-human clinical trials: A five point strategy for building a robust CMC dossier
생물학적제제로 최초 인체 대상(FIH) 임상시험을 수행하기 위해서는 이러한 복잡한 고분자 물질 특유의 과학적/규제적 문제들에 대한 깊고 미묘한 이해가 요구됩니다...

Webinar
Going global: The impact of US foreign trade zones on drug product manufacturing costs and timelines
오늘날과 같은 글로벌 경제에서는 세계 전역에서 의약품 원료를 소싱할 수 있습니다. 이런 원료 수입은 큰 비용을 수반합니다...

Webinar
The future of decentralized clinical trials in an evolving EU regulatory landscape
코로나19 팬데믹을 계기로 현장 중심 업무였던 임상시험을 완전 분산형 또는 하이브리드형으로 조정 가능함이 입증되었고 전세계적으로 분산형 시험이 임상연구를...

Webinar
Driving Supply Chain Productivity in Cell Therapy Clinical Trials
세포 유전자 치료제 임상 공급망 계획 시에 사전 위험을 식별하고 철저한 계획을 수립하는 방법을 알고 계신가요? 상업화를 준비할 때 데이터를 적극 활용하기 위한 최적의 방법을...

Webinar
Does Your Clinical Trial Design Satisfy the Needs of your Patients?
분산형 임상시험은 코로나19 팬데믹 이전에도 증가 추세에 있었습니다. 귀사의 임상시험에도 이 방식을 적용할 수 있을까요? 분산형 임상시험은 환자가 연구 기관을 방문하여 치료를 받을 필요...

Webinar
Conventional vs. Unconventional Spray Drying Strategies: Development to Commercialization
여러 원료의약품에서 발생하는 생체이용률 또는 결정화 문제를 해결하는 건조 방식에 대한 당사 업계 전문가들의 의견을 확인...

Webinar
Choosing the Right CDMO for Late Phase Clinical Trials
임상 단계를 거치면서 광범위한 역량과 풍부한 경험을 보유한 파트너와 협업해야 하는 이유와 이점에 대해 확인...

Webinar
Cell and gene therapy manufacturing in a post-covid world
포스트 코로나 시대, 세포 유전자 치료제 제조 최적화 방법
팬데믹으로 인해 세포 유전자 치료제 제조사는 원료 물질 부족, 무리한 제조 역량 사용, 과중한 공급망 물류 부담, 연구 및 임상 개발의 중단과 관련된 문제에 직면했습니다. 이러한 상황에서, 세포 유전자 치료제 제조사들은 치료제의 임상 및 상업화 성공을 위해 공정을 최적화, 간소화해야 한다는 점을 깨닫게 되었습니다...

Webinar
Building viral vector capacity and capabilities to realise the promise of gene therapies
바이러스 벡터 제품의 시장 출시에 따른 도전과제와 기회, 유전자 치료제의 약속을 실현하기 위한 Thermo Fisher의 생산 역량과 현황을 확인...

Webinar
Building a robust FIH biologics regulatory CMC package
후보물질이 임상시험 단계에 진입할 때는 반드시 임상시험 수행을 뒷받침하기 위한 서류를 준비해야 합니다. 이 서류의 필수 요소는 후보물질의 화학(Chemistry), 제조(Manufacturing), 품질관리(Controls)를 보증할 수 있는 CMC 패키지...

Webinar
Breakthrough Therapy Designation: Evaluation of trends and impact on CMC Strategies
혁신치료제 지정(BTD)은 FDA에서 심각하거나 생명을 위협하는 질병을 치료하는 신약의 개발과 심사 기간을 단축하기 위해 도입한 제도입니다...

Webinar
Manufacturing strategies for plasmid DNA in advanced therapy applications
세포 및 유전자 치료제 시장은 투자 급증과 IND 신청 증가를 겪으면서 이러한 획기적인 치료약을 개발할 때 사용되는 중요한 원료에 대한 규제 가이드라인과...

Webinar
An optimized approach to drug development
Thermo Fisher Scientific의 Quick to Care™ 프로그램을 활용하면 원료의약품과 완제의약품 개발, 수요 계획, 임상 공급을 단일 맞춤형 솔루션에 통합하여...

Webinar
Form and fit: Mobilizing integrated resources to transform complex small molecules into high-performing drugs
고객들이 개별 임상 단계에서 최대한의 혜택을 추구함에 따라 통합 서비스 계약 파트너가 제시하는 장점이 이러한 높은 수익을 현실화하고 있습니다...

Webinar
Key considerations when selecting a CDMO partner for cell therapy manufacturing
세포 및 유전자 치료 시장은 가속화된 시장 승인, 역대 최고의 투자, 견고한 치료 파이프라인, 긍정적인 임상 결과를 경험하고 있으며, 이에 따라 제조 기술의 속도, 규제 노하우, 혁신의 필요성이 커지고 있습니다...

Webinar
Advanced therapies and large molecule investments
바이오의약품, 세포/유전자 치료제, 완제의약품 개발 및 생산 역량 강화와 임상 공급망 확대를 위한 당사의 투자 현황을 확인...

Webinar
Addressing technical challenges in development drug products: A CDMO perspective
이 웨비나에서는 Thermo Fisher Scientific이 위탁 개발 중 기술적 난제 해결을 어떻게 지원해왔는지 자세히 소개합니다. 당사의 솔루션이 효과적인 의약품 개발과...

Webinar
Addressing scalability challenges in the development and manufacture of gene therapies
희귀 질환, 암, 공공 보건 문제를 해결하기 위한 유전자 치료, 유전자 변형 세포 치료 및 백신에 바이러스 벡터 사용이 승인되었습니다. 이러한 치료제들은 빠른 속도로 상업화를 향해 나아가고 있으며 종종 다량의 벡터가 필요한 경우가 있습니다...

Webinar
Accelerating innovative therapies to patients in a post pandemic world
연구부터 출시까지, 성공적인 상업화를 위한 세포 치료제 개발 과정, 팬데믹 이후 세계의 mRNA 백신, 기술 및 공급망 등 포스트 코로나 시대 세포 유전자 치료제 개발 최적화에 대한 내용을 확인하세요

Webinar
Accelerate late-phase drug development with continuous manufacturing
경구제 공정 개발은 여러 단계에 걸쳐 진행됩니다. 일반적으로 최종 스케일업 공정은 상용화 승인을 신청하기 직전까지 결정되지 않습니다. 연속 생산은 3상 개발과 임상 생산에 매우 적합하며, 고품질의 결과물을 제공하고 API 사용량을 절감합니다...

Webinar
A toxicologist’s viewpoint on developing oncology drug products
항암제 분야가 성장하면서 제약회사는 빠르고 민첩하게 구명 치료를 개발하고 제조해야 한다는 압력을 받고 있습니다. 암 증가율은 환경적 요소, 라이프스타일 선택, 인구 고령화로 인해 치솟고 있습니다...

Webinar
Regulatory and cost implications for switching injectable delivery formats
다양한 주사 제형 옵션이 있으므로, 한 가지 형태의 고분자로 시판하고 나서 나중에 투여 형태를 바꾸기로 결정하는 것은 드문 일이 아닙니다. 이러한 일반적 관행은 종종 비용 절감 솔루션 및 공급 체인 연속성을 제공할 뿐만 아니라…
Article
Prepping for commercialization through supply chain logistics
세포 및 유전자 치료약 시장이 급속하게 확대됨에 따라 혁신가들은 필요한 환자들에게 안전하고 효율적으로 제품을 전달할 수 있는 신뢰할 수 있는 공급망 솔루션을 요구하게 될 것입니다...
Article
Understanding the roles of solid-state characterization and crystallization within the product lifecycle
고형 활성약물성분(API)의 물리적 속성은 분리, 정제, 형태 제어에 이용되는 결정화 공정을 결정하고, 효과적인 제형 개발로 이어질 수 있게 합니다. ..
Article
A comprehensive approach to improving solubility and bioavailability Spray drying
임상 개발 중인 신약의 80% 가량에 용해도 문제가 있으므로 제품 제형과 제조 시 특별한 주의가 필요합니다...
Article
Ancillary supplies clinical trial must haves that require early planning
필요용품 공급은 모든 임상시험의 필수 요소입니다. 그러나 이 용품들은 종종 부차적인 것으로 인식되곤 합니다. 필수용품 관리가 IMP만큼 중요한 이유와 의뢰자가 임상 필수용품 공급 초기 계획에 주의를 기울여야 하는 이유...

eBook
Protecting tomorrow: Supporting sustainability in the pharmaceutical and biotech industries
기후변화는 한계점에 도달해 있고 우리는 더 이상 지체할 시간이 없습니다. 상호 지속 가능한 여정을 성공적으로 맞이하기 위해서는 협업이 필수적입니다.

eBook
Cell and gene therapies in the US vs. the EU: Top five areas of differentiation
세포 유전자 치료제를 비롯한 바이오 의약품 분야에서 미국과 유럽의 인허가 절차는 단순한 관할 지역 차이를 넘어 크게 다릅니다.

eBook
Using an Approved Phrase Library
Find out how to avoid delays in translating and approving clinical labels that can prevent clinical trials from starting on time and threaten to derail development timelines.

eBook
The Challenge of Keeping Cool: End-to-End Temperature Management for the Clinical Supply Chain
의약품을 생산하여 환자에게 전달하는 과정에서는 엄격한 온도 지침을 준수하여 제품의 효능을 유지해야...
eBook
Managing Temperature Excursions: Key Pointers That Must Be Addressed
공급망 전반에 걸쳐 콜드체인 의약품의 무결성을 유지하려면 민감한 임상시험용 의약품(IMP)의 포장, 처리, 보관, 유통부터 임상시험 기관에 이르기까지 전 과정에 걸쳐 엄격한 공정과 최고 수준의 콜드체인 전문 지식이 필수적…

eBook
Reasons for Building an Approved Phrase Library
임상시험에 참가하는 국가의 수가 늘어나면서 임상시료 라벨 제조 문제가 중요해지고 있습니다. 평균 약 120영업일이 소요되는 임상용 라벨 번역과 승인이 지연되면 임상시험을 적시에 시작하지 못하게 되어 개발 일정이 지연될 수 있습니다...

eBook
Cold Chain Industry Trends
Take a look at the importance of a robust supply chain and get planning recommendations for pharma companies scaling up to global vaccine trials.

eBook
Cold Chain Qualification: 5 Questions You Must Ask When Shipping Biologics
Download the eBook to learn about the different types of considerations when shipping biologics.

eBook
Clinical Trial Packaging Solutions: Balancing Cost, Time and Quality
매년 10,000개 이상의 패키징 관련 일자리를 창출하는 4,500건 이상의 임상시험을 지원하다 보면 확실해지는 한 가지 사실이 있습니다. 그것은 바로 경험의 축적입니다. 이는 시간, 비용, 품질 사이에 최적의 균형을 맞추고자 노력하고 있는 임상 의뢰자들에게 소중한 통찰이...

eBook
Cell Therapy Logistics: Advanced Therapy
Overview key considerations for developing a successful logistics strategy for the management of cell-based material.

eBook
Beyond Procurement: Taking a Strategic Approach to Comparator Drug Sourcing
대조약 시장이 성장함에 따라 임상 의뢰자와 임상 공급 업계의 어려움도 커졌습니다. 최고 품질의 대조약을 짧은 리드타임에 공급하는 것은 매우 어려운 일일 수 있습니다. 대조약 소싱 어려움을 극복하기 위한 전략...

Fact Sheet
Advanced therapy supply chain solutions
Thermo Fisher Scientific은 맞춤형 패키징과 라벨링부터 환자 검체, 약품, 세포주의 콜드체인 보관까지 수백 번의 세포 및 유전자 치료제 시험을 거쳐 쌓은 입증된 경험을 바탕으로...

Fact sheet
Global regulatory services backed by industry experts
잡한 규제 환경을 헤쳐나가는 것은 제품 수명주기 전반에 걸쳐 중요한 문제입니다. Thermo Fisher Scientific은 여러 가지 유연한 규제 솔루션을 통해...

Fact sheet
ATLAS (Alternative Translation and Label Approval System)
다국어 라벨 텍스트 개발을 자동화하고 최적화하는 검증된 임상 라벨 번역 관리 서비스인 독점적인 웹 기반 대체 번역 및 라벨 승인 시스템(ATLASSM)에 대해 알아보십시오.

Fact sheet
Sample Fulfillment & Distribution Overview
Patheon Logistics는 유통을 최적화하고 운송 효율을 높이면서 제품 안정성과 업계 표준 준수를 보장합니다. 고객사의 담당자 직접 배송 및 의료인 직접 배송 샘플링 프로그램을 지원하기 위한...

Fact sheet
Patheon™️ Quick to Clinic™️ Viral Vector Services
렌티바이러스와 AAV 제조를 위해 최적화된 IND 준비 플랫폼 공정을 갖춘 통합 개발 프로그램으로, 개발 단계에서 발생할 수 있는 위험 요인을 관리하고 개발과 제조 시간을 줄여줍니다...

Fact sheet
CMO Scorecard
Patheon pharma services는 지난 10년간 가장 많은 NDA 승인 의약품의 개발 및 공급을 지원한 CDMO입니다. 당사는 광범위한 글로벌 네트워크를 통해 전체 임상 단계에 걸쳐 모든 의약품의 개발, 생산에 대한 통합적인 서비스를 제공...

Fact sheet
Specialty Courier Services Overview
발송인 추천부터 수입/수출 통관 촉진에 이르기까지 모든 것을 포함하는 최적의 전문 택배 솔루션을 설계하는 당사의 능력에 대해 자세히 알아보십시오.

Fact sheet
Preferred Carrier Management Overview
생명 과학 표준 발송물이 온도 제어 및 검증된 트레일러를 포함하도록 고유하게 특화된 Preferred Carrier 네트워크로 어떻게 제한되는지 알아보십시오...

Fact sheet
Continuous Manufacturing: The Alternative to Batch Manufacturing
제약업계는 갈수록 커지는 품질, 가격, 생산성 압박을 직면하고 있습니다. 경구제를 제조하는 기존의 배치형 제조 공정은 솔루션이 될 수 없습니다...

Fact sheet
Commercial Packaging Overview
임상 및 상업용 공급망에서 여러 업체를 컨택하면 끊임없이 문제가 발생합니다. 제품이 어디에 있는가? 사양에 맞게 패키징이 가능한가? 소량 취급도 가능한가? 등을 벤더마다 체크해야 합니다...

Fact sheet
Direct-to-Patient Services
품질을 저해하지 않고 시장에 출시하는 속도를 확보하기 위해서는 임상시험을 가속화하는 것이 해결 방안일 수 있습니다. 그러나 기존의 임상시험 모델은 이러한 목표에 적합하지 않습니다...

Fact sheet
Engineered Solutions for oral solid dose product development
엔지니어드 솔루션은 원료의약품, 관련 제조 공정, 제품, 생물의약품 속성, 이들 각 요소가 제품의 성공에 미치는 본질적 연관 관계와 중요성을 살펴봄으로써...

Fact sheet
mysupply Platform: An End-to-End Digital Supply Chain Platform
제품 Lifecycle 전반에 걸쳐 가시성 높은 데이터로 생산 관리를 지원하는 end-to-end 디지털 공급망 관리 플랫폼...

Fact sheet
Fill/Finish Services for Viral Vectors
바이러스 벡터 및 백신의 충전/완제 생산 역량을 필요로 하는 바이오 벤처 기업, 중견 기업, 대기업 등 모든 규모의 기업과 연구 기관에 충전 및 마감 공정(Fill and Finish) 서비스를 제공...

Fact sheet
Quadrant 2™ Solubility and Bioavailability Improvement Solutions
대다수의 신약 후보 물질은 용해도와 생체 이용률이 낮다는 문제를 안고 있으며 이는 임상 진입 전에 반드시 해결되어야 하는 과제입니다. Quadrant 2® 는 컴퓨터 모델링을 통한 인실리코(in-silico) 제형 예측으로 초기 제형 개발 단계를 지원...

Fact sheet
Clinical Supply Optimization Services
성공적인 임상 시험의 요건은 다양합니다. 임상 시험 공급망의 효과적인 계획과 관리는 임상 성공의 주요 요건입니다....

Fact sheet
API (Small Molecule) Overview
전 지역에서 경쟁력 있는 API 아웃소싱 옵션이 있는 상황에서 복잡성에 관계없이 프로젝트에 맞는 파트너를 선택하는 것이 어려운 일입니다...

Fact sheet
Biopharmaceutical consignment production service overview
구조가 복잡한 고분자 의약품의 개발 및 생산에 대한 기업의 위험 부담과 비용은 기하급수적으로 증가하고 있습니다. 또한 기업들이 개발과 제조에 있어 경쟁 우위를 점하기가 더욱 어려워지고 있습니다...

Fact sheet
Clinical Ancillary Management Services
Thermo Fisher Scientific의 임상 보조용품 관리(CAM) 서비스는 공급망 전문가팀이 고객사와 협업하면서 임상시험 셋업 계획 초기부터 임상시험 이행, 임상 종료까지...

Fact sheet
mRNA Manufacturing Services
써모 피셔 사이언티픽은 바이오 제약 산업과 서비스에 대한 깊은 이해를 바탕으로 당사의 역량을 통합해 mRNA 치료제의 개발부터 상업생산에 이르기까지...

Whitepaper
Hot melt extrusion: Improving solubility of poorly soluble compounds
몇몇 제약사는 개발 첫날부터 제재 확장성을 설계하고 임상 3상 대량 생산에 완벽히 대비하기도 합니다. 그러나 대다수는 정제, 바이알 등 형식에 관계 없이 대부분의 경우 3상에서 불가피하게 예상하기 힘든 문제에...

Whitepaper
Technology transfers: Best practices for optimizing success and mitigating risk
스케일업 또는 개발에서 제조 단계로 전환하기 위해 생산 사이트를 변경할 때에는 기술 이전이 필수입니다...

Whitepaper
Safety first: Controlling occupational exposure in oncology drug development
경구용 항암제의 경우, 활성 의약품 성분(API)이 고효능이고 소량만 복용해도 독성이 있을 수 있는 경우가 많습니다...

Whitepaper
Advancing drug development using in silico modeling
데이터는 지식이고, 지식은 곧 힘입니다. 그러나 지식은 실행 가능할 때만 힘이 됩니다. 예측 모델링은 견고한 신약 개발 및 제조 플랫폼을 개발하는데 도움이 될 수 있습니다..

Whitepaper
EU Clinical Trial Regulation 2022: Understanding the impact on clinical research in Europe
도입된 지 8년이 된 EU 임상시험규제(CTR) 2022가 본격적으로 적용되면서 EU 회원국과 유럽경제공동체(EEA) 소속 국가의 임상시험 실시 규제 환경이 급변하고 있습니다...

Whitepaper
Small molecule orphan drugs: Balancing financial incentives and complex challenges
희귀의약품을 개발중인 기업이 인허가 과정을 통과하려면 임상 돌입 속도와 비용 간의 균형을 맞춰야 합니다...

Case study
Using Quality By Design For Process Development And Scale-Up Of A Novel ALS Drug Product
3상 임상의 주요 중간 목표 기한이 다가옴에 따라 Amylyx와 Thermo Fisher는 공정 개발과 등록 배치의 스케일업을 위해 개발 및 제조 요소 몇 가지를 고려해야 합니다...

Case study
Cost Savings and Speed: The Untapped Value of a Single-Source Solution
소규모 신생 제약사는 한정된 예산과 자원으로 빠르게 움직여야 후보물질을 FIH 그리고 그 다음 POC 단계로 진행시킬 수 있습니다. 내부에는 적은 수의 인력만을 두고 여러 벤더에 주로 외주를 주기도 합니다...

Case study
Direct to Representative Sample Distribution Services
제약사는 다양한 마케팅 채널을 사용하여 처방 의약품을 홍보합니다. 미국의 경우, 이러한 채널 중 하나는 제약 영업 담당자가 지역 내의 의료 종사자에게 샘플을 '손수' 가져가는 것입니다...

Case study
High Precision Syringe Labeling
환자 안전은 모든 임상시험에서 가장 큰 우려 사항이지만, 특히 주사기가 관련된 경우 더욱 그렇습니다. 정확한 양의 약물이 매번 투약될 수 있도록 투여 방법이 정확해야 합니다....

Case study
Flexibility Enables Managing Large Leaflet on Automatic Packaging Line
한 다국적 제약사는 이탈리아 페렌티노에 있는 Thermo Fisher Scientific 사이트에 제품 정보를 담고 있는 리플렛 사이즈 변경 문제를 해결해줄 수 있는 지 문의했습니다...

Case study
Global Ultra-Cold Clinical Trial Logistics
과제: 세포 기반 치료제를 생산하는 새로운 미국 소재 바이오 제약회사는 임상시험 범위를 확대하고 중국 베이징에 있는 환자를 포함하도록 환자 풀을 확장했습니다...

Case study
How to Select the Best Courier Around the Globe
세계 최고의 생명공학 기업 중 하나로 글로벌 입지가 지속 성장하면서 파이프라인도 성장했습니다. 이러한 성장과 함께 공급망 관리 문제도 발생했습니다....

Case study
Total Transportation Management Saves Company $10.2M
많은 임상시험 파이프라인을 갖고 있는 다국적 제약회사는 완벽하게 관리되는 통합적인 운송 전략이 필요했습니다. 이 회사는 임상시험에 필요한 배송을 아웃소싱함으로써 기업이 가장 잘하는 일...

Case study
The Criticality of API CDMO Selection: Insights from a Client
비용, 기술 역량 등을 포함한 한정된 자원 문제에 직면한 바이오파마 스타트업은 경험이 풍부한 파트너의 지원이 필요합니다...

Case study
We work together to meet global demand for COVID-19 vaccines
전 지구적 코로나19 팬데믹은 지난 2년 동안 전 세계를 근본적으로 뒤흔들어 놓았습니다. 공중보건 및 경제 위기를 초래한 팬데믹은 2차 세계대전 종전 이래 문명사회에 대한 최대 위협으로 기록되었습니다…

Case study
Delivering a large-scale product at a rapid pace
Thermo Fisher Scientific Pharma Service 팀은 고객이 다섯 단계의 까다로운 화학물질을 도입, 제조, 검증하여 3상 임상 개발 프로그램을 지원했습니다...

Brochure
Pharma Services Overview
Patheon (파테온)은 모든 규모의 제약 및 바이오 기업을 위한 end-to-end 의약품 위탁 개발 및 생산 서비스를 제공합니다. 전 세계 65개 이상의 글로벌 생산 네트워크를 기반으로...

Brochure
Sample Fulfillment & Distribution Overview
Patheon Logistics는 유통을 최적화하고 운송 효율을 높이면서 제품 안정성과 업계 표준 준수를 보장합니다. 고객사의 담당자 직접 배송 및 의료인 직접 배송 샘플링 프로그램을 지원하기 위한...

Brochure
Solid and Sterile Dose Form Overview
Thermo Fisher Scientific이 제공하는 다양한 표준형 및 특수 경구제 옵션을 확인하실 수 있습니다. 혁신적인 제형 조합과 방출제어 기술도...

Brochure
Small molecule API solution: Taking a big picture approach
전 지역에서 경쟁력 있는 API 아웃소싱 옵션이 있는 상황에서 복잡성에 관계없이 프로젝트에 맞는 파트너를 선택하는 것이 어려운 일입니다...

Brochure
Large molecule development and manufacturing: Comprehensive offering enabling speed and flexibility
당사의 통합형 및 맞춤형 서비스를 활용하면 복잡성을 제거할 수 있고 고분자 의약품을 보다 적은 비용과 낮은 위험으로 더 빠르게 출시할 수 있습니다...

Brochure
Oral solid dose – Access a range of flexible drug development & manufacturing solutions
복잡한 규제 환경 극복과 분석 데이터, 공정 개발 및 최적화, 정시 전달은 모두 경구제 성공에 있어 필수적인 요소입니다...

Brochure
Steriles drug development and manufacturing: Flexibility and optimization
지난 5년간 무균 주사제 분야가 10% 급성장함에 따라 개발 및 제조 측면에서의 생산성 확대와 혁신적인 솔루션에 대한 필요성이 대두되었습니다. 복잡한 규제 환경 대응, 분석, 공정 개발 및 최적화, 정시 생산은 모두 무균 주사제의 성공적인 생산에 필수적인 요소입니다...

Brochure
Leading Viral Vector CDMO Services for Cell and Gene Therapies
Viral Vector Services (VVS)는 세포 유전자 치료제 개발을 위한 바이러스 벡터의 공정 및 분석법 개발부터 임상 및 상업 생산까지 전 과정을 지원하는 end-to-end 바이러스 벡터 CDMO 파트너 서비스...
Brochure
Biopharma Capabilities Overview
Thermo Fisher Scientific은 고품질의 서비스와 지속적인 혁신을 기반으로 의약품 개발과 생산 전과정에서 고객을 성공으로 인도합니다...

Brochure
Global clinical supply solutions for every trial delivering the right drug to the right patient, on-time, in-full, and without compromise
견고한 공급망 전략을 안내하고 공급망 관리에 도움을 줄 수 있는 파트너를 찾는 것은 회사의 신약 개발 만큼 중요한 과정입니다...

Brochure
Biologics Overview: Flexible Biomanufacturing Solutions
고객의 후보물질은 삶을 변화시키고 미래를 만들어갈 힘을 갖고 있습니다. Thermo Fisher Scientific은 고객의 성공을 가속화하고 동시에 최고 품질을 유지할 수 있도록 지원합니다...
Brochure
Clinical Trial Supply Solutions
Thermo Fisher Scientific은 30년이 넘는 시간 동안 모든 고객이 임상 시험 수행 시 유연성, 합리적인 비용, 위험 최소화를 위한 접근 방식을 모두 갖춘 종합적인 임상 개발 계획을 수립할 수 있도록 지원해 왔습니다...

Brochure
Developing a CMC and regulatory roadmap for your molecule’s lifecycle
약물 개발을 시작할 때부터 제대로 된 전략을 수립하는 것이 각 개발 단계를 거쳐 상업화 단계로 진행되는 동안 시간 및 비용 절감에 도움이 됩니다…

Brochure
Pharma Services EMEA network, integrated network from molecule to medicine
브로슈어를 통해 복잡한 EMEA 지역 내 신약 개발, 제조, 임상 개발 문제를 해결하는 당사의 헌신적인 서비스와 솔루션에 대해 자세히 확인하세요.
Brochure
Transportation Solutions for Cell and Gene Therapy Supply Chains
세포 및 유전자 치료제 유통과 공급의 최우선과제는 의약품의 무결성을 유지하면서 목적지까지 정시 도착과 보관 온도 조건을 준수하는 것입니다. 세포 유전자 치료제는 운송 시 속도, 온도, 무결성을 유지해야...

Webinar
Flexible regulatory pathways and key CMC considerations to commercialize cell and gene therapy products
해당 웨비나에서는 세포 및 유전자 치료제의 인허가 절차를 안내하고 이에 대처하는 방법을 설명합니다.

Webinar
Accelerating your biologic’s development: From lab, to clinic, to market
Thermo Fisher Scientific이 신생 바이오기업의 임상 및 출시 기간 단축을 어떻게 지원하는지 알아보십시오. CDMO는 이제 성공적인 의약품 개발을 위한 필수 협력 파트너로 인식되고 있습니다.

Webinar
Preparing your biologic for commercialization: strategies to reduce risk and optimize outcomes
후기 전략을 잘 선택하여 일정과 생산량을 최적화하는 한편 변화하는 시장 상황에 대처할 수 있는 유연성을 유지하는 일은 바이오의약품을 개발하고 상용화하는 기업들에게는 큰 도전과제입니다.

Webinar
Risk assessment strategies in oral solid dosage development and manufacturing
신약 개발의 각 단계에 접어들 때마다 스케일업을 시도할 때 조심해야 할 수많은 항목이 있습니다. 웨비나에서 스케일업을 위한 최상의 전략을 확인하세요.

Article
Patheon(파테온), “초기 개발 소요 시간 단축하는 혁신 솔루션 제공… 한국 바이오 제약 기업 성장 돕는다!”
에이빙 뉴스의 ‘Post 바이오코리아 특집 : 한-호주 간 비즈니스 기회를 잡아라’ 기사를 통해 당사의 호주 브리즈번 생산시설과 호주 정부기관과의 협업에 대해 확인하세요.

Article
성공적 mRNA 개발 CDMO 역량 5가지
바이오스펙테이터에 당사 Subject Matter Expert인 Vincenza Pironti 박사의 칼럼이 게재되었습니다. 자세한 내용은 기사를 통해 확인하세요.

2022 Bio Korea
Thermo Fisher Scientific이 2022 BIO KOREA에 참여합니다.
일시: 2022년 5월 11일 ~ 13일
장소: 코엑스
부스번호: K10 Thermo Fisher Scientific

Biologics Manufacturing Korea 2022
Thermo Fisher Scientific이 BMK 2022에 참여합니다.
일시: 2022년 6월 29일 ~ 30일
장소: 인천 송도컨벤시아

2023 Bio Korea
Thermo Fisher Scientific이 2023 BIO KOREA에 참여합니다.
일시: 2023년 5월 10일 ~ 12일
장소: Coex Seoul
부스번호: N4 Thermo Fisher Scientific

Video
Quick to Clinic™ for Oral Solid Dose
Learn how Reneo Pharma was able to get to clinic in 14 weeks from delivery of their API.

Video
United with process & partnership
An experienced CDMO partner with an extensive network makes the difference when it comes to scale-up, tech transfer capabilities, and validation.

Video
United with capabilities & compassion
Is it possible to bring your molecule to medicine without a brick and mortar site and no testing labs or distribution capabilities?

Video
Solved with intelligence & imagination
When a client faced a complicated packaging challenge, Thermo Fisher Scientific’s Lead Account Manager went to the drawing board, literally.

Video
Made with N-of-1 & all-for-one
What seemed impossible became achievable when Thermo Fisher Scientific manufactured and released a one-of-a-kind treatment in a record timeline of 30 days.

Video
You Inspire Us Every Day
Michel Lagarde, Executive Vice President, Thermo Fisher Scientific, discusses why Pharma Services employees are so passionate about partnering with small and emerging biopharma clients.

Video
Vectoring In — Viral Vector Video Series
Preparing cell and gene therapies for regulatory submission
When preparing to ramp up late stage manufacturing for commercialization, understanding key critical to quality parameters will help prepare your therapy for regulatory submission.

Video
Thermo Fisher Scientific & CSL Enter Strategic Partnership to Provide Best-in-Class Pharma Services
We are pleased to announce that Thermo Fisher Scientific and global biotechnology company CSL have entered into a strategic partnership to help meet the growing demand for biologic therapies while also accelerating CSL’s broader manufacturing objectives.

Video
The Journey from Molecule to Medicine
It all starts with your discovery. A molecule, small or large, that needs a partner. Let Patheon be that partner.

Video
The “Eureka Moment” That Helped a Small Company Make a Big Impact
Tony and the team at Thermo Fisher Scientific understand the needs of small and emerging companies.

Video
Step Beyond with Thermo Fisher Scientific
To serve science, Thermo Fisher Scientific needs to stay ahead of it. To be the world leader in serving science, we need to anticipate customer needs.

Video
Solved With: Capacity and Compassion
Learn how Thermo Fisher Scientific was able to help this company solve one of their biggest challenges in the drug development process.

Video
Patheon’s Softgel Expertise
Learn more about the Softgel expertise at Patheon, by Thermo Fisher Scientific.

Video
Our Formulation of Heart and Science is the Key to Our Success
At Thermo Fisher Scientific, everything we do is made with the right balance of heart and science. Find out how Angie and our Fisher Clinical Services team went above and beyond to overcome tough obstacles in order to ensure thousands of patients around the globe received life-saving medications.

Video
One Bottle of Hope for One Amazing Kid
Meet Gavin and his mom, Nicole. Learn how Gavin’s battle with a brain tumor led him to the bottle of hope that allowed him to be a kid again.

Video
Made For: Hope for ALS
Learn how Amylyx Pharmaceuticals partners with Thermo Fisher Scientific to bring hope to patients with ALS.

Video
How Viral Vector Technology Is Rapidly Scaling up to Enable One Miracle After Another
The promise of viral vectors has been pursued for over two decades. But in the last few years, this transcendent technology that’s targeting over 200 diseases has finally started to create real treatments and possible cures.

Video
How two college kids took a dorm-room idea all the way to clinical trials.
Watch the video to learn why companies like Amylyx are choosing to partner with Thermo Fisher Scientific for their outsourcing needs.

Video
How Thermo Fisher is Battling COVID-19
We are proud to be on front lines arm-in-arm with our clients, fighting COVID-19. Together, we will win. Every patient matters.

Video
How Spring Break Had to Wait a Little Longer, So a Baby Could Have a Better Shot
After a long night of packing for her family’s spring break vacation, Holli woke up at 4:40 AM Saturday morning. She noticed a text message on her phone.

Video
How Speaking a Third Language Helped Win the Race to Market
Several Chinese pharmaceutical companies were competing to be first to market. And second place didn’t matter.

Video
How One Split-Second Decision Made a Difference for Thousands of Patients
In the world of drug manufacturing not all life-saving decisions happen in the lab. Sometimes it’s a lot closer to delivery that key moments require fast thinking.

Video
How One Brave Patient Went from the Heart Transplant List to Climbing the Mountain Trails
“It felt like getting kicked in the chest by a horse.” That’s how Linda described the shock she received from her implanted defibrillator when she had one of her yearly “mini heart attacks.”

Video
How Leveraging a Global Network Delivered Big Results
Mike’s team in Bend, OR was only supposed to optimize the spray drying process for a particular medication while another, much larger, facility would handle the large-scale manufacturing. However, the other facility’s equipment wouldn’t be available in time to meet the client’s aggressive IND filing schedule.

Video
How Can Pharma Companies Save up to $45M in Early Drug Development?
Patheon’s Jennifer Therrien discusses findings of a Tufts Center for the Study of Drug Development (CSDD) study that reveals how pharmaceutical companies using a single-source outsourcing partner can achieve a net gain of $45 million in early drug development.

Video
How a Viral Vector Got the Boost It Needed to Start Defeating an Incurable Disease
Duchenne’s Muscular Dystrophy is an incurable disease that mostly affects boys. It results from a gene defect that prevents those affected from developing normal muscle structure and function.

Video
How a Small Non-profit Teamed up with a Global Manufacturer to Bring Their ALS Drug to Clinical Trials
Before she lost her own battle to ALS in 2003, Jenifer Estes started Project ALS, raising over $17 million dollars for the non-profit in hopes of a breakthrough in the fight against Lou Gehrig’s disease. As her family continued the quest, in 2019, they got a hit with a new compound that seemed to stop or even reverse motor nerve damage.

Video
How a Family’s Perseverance Helped Them Reach a Seemingly Unattainable Goal
As members of the United States Air Force, Lauryn and Chris were as healthy and fit as a young couple could be. But in the world of genetics, all it takes is a couple of proteins lining up in the wrong way, and things can change in a hurry.

Video
How a Combination of Medicines Helped One Man Take Back His Life, and Inspired Us All
On a quiet Sunday afternoon, Pete and his wife Lisa took a moment to sit on a porch swing and relax. A small piece of wood holding the chain broke and they fell backwards causing Pete to break his neck.

Video
Going the Extra Mile is Part of Our DNA
The stakes were high for a client who needed material for a novel drug to in order to proceed to clinical trial. With millions of dollars on the line, Jeff’s team had one shot to develop a process to scale up or risk failing the batch.

Video
Flexible Oral Solid Solutions From Early Development to Commercial Manufacturing
Thermo Fisher Scientific provides a range of flexible oral solid dose solutions that can help you easily address your small molecule’s unique needs and challenges from early development to commercial manufacturing.

Video
Fisher Clinical Services & Patheon: Better Together
Leon Wyszkowski, President, Commercial Operations, discusses the value of Patheon and Fisher Clinical Services joining together to provide integrated solutions for our clients.

Video
Delivered With: Partnership and Passion
Learn why this supply chain executive calls Thermo Fisher Scientific the CDMO partner of choice.

Video
Delivered With: A Positive Client Experience
Learn how a global CDMO never loses sight of what’s important to our clients.

Video
Continuous Manufacturing in Patheon’s Greenville, North Carolina Site
We are the first CDMO to build and manufacturing client product on a continuous manufacturing line. The suite, located at the site in Greenville, North Carolina, is custom built to utilize the best equipment available.

Video
Collaboration and Knowledge Transfer Equals Success for Patients
Collaboration is an important part of our culture at Thermo Fisher Scientific and it starts with our people. Emily and Jessica are a great example of how a mentor relationship facilitates the transfer of critical knowledge to achieve long-term results.

Video
Clinical and Commercial Manufacturing for Oral Solid Dose in Greenville, North Carolina
Clinical and Commercial Manufacturing for Oral Solid Dose in Greenville, North Carolina.

Video
The Medicine Maker, Chapter 6: Leading Industry Transformation
Video Webinar with The Medicine Maker, Chapter 6: Leading Industry Transformation.

Video
The Medicine Maker, Chapter 5: Planning for Insourcing or Outsourcing
Video Webinar with The Medicine Maker, Chapter 5: Planning for Insourcing or Outsourcing.

Video
The Medicine Maker, Chapter 4: Measuring Forecast Accuracy
Video Webinar with The Medicine Maker, Chapter 4: Measuring Forecast Accuracy.

Video
The Medicine Maker, Chapter 3: Identifying Volatile Forecast Variables
Video Webinar with The Medicine Maker, Chapter 3: Identifying Volatile Forecast Variables.

Video
The Medicine Maker, Chapter 2: Overcoming Commercial Launch Challenges
Video Webinar with The Medicine Maker, Chapter 2: Overcoming Commercial Launch Challenges.

Video
The Medicine Maker, Chapter 1: Understanding Industry Shifts
Video Webinar with The Medicine Maker, Chapter 1: Understanding Industry Shifts.

Video
Benefits of Working with Patheon Softgels
Kaspar van den Dries, Ph.D. discusses the benefits of working with Patheon for your softgels project.

Video
API to Clinical Trial Distribution: The Value of Patheon’s Integrated Offering
From preclinical to clinical trial distribution to commercial manufacturing, we can help you through the entire process.

Video
Advantages of Softgel Technologies with Tony van Bijleveld
Tony van Bijleveld, Vice President, Sales, discusses the benefits of the recent Thermo Fisher Scientific acquisition, as well as Softgel expertise & technology to enable innovative customer solutions for patients.

Video
Advantages of Softgel Solutions for Developers
Kaspar van den Dries, Ph.D. discusses the many advantages for drug developers who choose Softgels.

Expert
Monica Commerford, PhD
Head of Viral Vector Services Regulatory Affairs
Scientific Expertise:
- Cell and gene therapy CMC expert
- Microbiology and molecular genetics
- Microbial control, Product Quality microbiology, and Sterility Assurance for investigational new drug and marketing applications
- Developing, editing and reviewing regulatory submissions, including reviews as a health authority assessor
- Regulatory intelligence and interpreting regulatory guidance
Focus Area
Viral Vector Services
Credentials
Sallie Rosen Kaplan Post-Doctoral Fellow, National Cancer Institute, National Institutes of Health
Doctor of Philosophy in Microbiology and Molecular Genetics from Harvard University
Bachelor’s degree in Biochemistry and Molecular Biology from Michigan State University

Expert
Kevin Shea
Senior Label Program Director
Expertise:
- Clinical supplies, with expertise in clinical labels, label translation and regulatory services
- Customer oversight/maintenance
- Customer liaison
- New business
- Bid defences
- Issue Resolving
- Webinars, Workshops, Presentations, and Whitepapers
- Trade shows
- Escalations
- Technical support
- Training, Quality and Audit support
Focus Area
Clinical label services
Clinical trial services
Credentials
Bachelor's Degree, Hobart College

Expert
Anil Kane, PhD, MBA
Senior Director, Global Technical and Scientific Affairs
Scientific Expertise:
- Product development
- Small molecules
- Pre-formulation, clinical development to lifecycle management
- Scientific strategic planning/problem solving
- Early development
- Clinical supplies
- Technology transfer
- Supporting chemistry, manufacturing, and controls documentation
Focus Area
Formulation and development
Credentials
Doctor of Philosophy from the University of Bombay, India
Master of Business Administration from Richard Ivey School of Business, University of Western Ontario, Canada
Post Doctoral research at the University of Cincinnati

Expert
Jennifer A Head, PhD
Manager of Regulatory Affairs, Microbial Manufacturing Services (MMS)
Scientific Expertise:
- Regulatory strategy for marketing of controlled substances, new molecular entities, and new chemical entities
- Regulatory intelligence and interpretation of regulatory guidance
- Developing, editing, and reviewing regulatory submissions
- Cell and gene therapy CMC expert
- Microbiology and immunology
- Viral pathogenesis
- Medical technology
- Central nervous system and oncology pharmaceuticals
Focus Area
Biologics
Credentials
Jean B. Kempner Postdoctoral Fellow and Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant recipient, Doctor of Philosophy in Microbiology and Immunology from University of Texas Medical Branch, Bachelor of Science degree in Medical Technology from University of Texas MD Anderson Cancer Center School of Health Professions, Bachelor of Science degree in Biology from Texas A&M University Corpus Christi

Expert
Douglas Hausner, PhD
Senior Manager, Continuous Manufacturing
Scientific Expertise:
- Continuous manufacturing
- Process analytical technology
Focus Area
Commercial manufacturing
Credentials
Doctor of Philosophy in physical chemistry from Temple University
Bachelor’s degree in chemistry from the University of Delaware

Expert
Richard Snyder, PhD
Vice President, Science and Technology
Scientific Expertise:
- Oral solid dosage forms, tablets, hard shell capsules, and softgels
- Virus biology
- Viral vector development
- cGMP viral vector manufacturing & analytical technologies
- Viral vector mediated gene transfer
- Development of novel viral vector-based human gene therapies
Focus Area
Viral Vector Services
Credentials
Post-doctoral fellow at Johns Hopkins University School of Medicine
Doctor of Philosophy in microbiology from The State University of New York at Stony Brook
Bachelor’s degree in biology from Washington University in St. Louis

Expert
Ronald Perry
Senior Manager, R&D
Scientific Expertise:
-
Formulation, technical transfer, and process optimizations for:
- Oral solid dosage forms, tablets, hard shell capsules, and softgels
- Oral liquids (solutions, suspensions, extended release suspension)
- Transdermal systems
- Sterile injectables
- Ophthalmic
- Medical device
- Aerosol products
- Packaging
- Creams and ointments
- Quality assurance experience including site auditing (domestic and international)
- Pharmacy/pharmaceutical consultant services ad libitum
Focus Area
Softgels
Credentials
Pre-med/pharmacy degree from Indiana University South Bend, IN
Bachelor of Science, Pharmacy from Purdue University School of Pharmacy in West Lafayette, IN
Studied diabetes education and anticoagulation therapy
Immunization certified
Registered Pharmacist (R.Ph.)

Expert
Gordon Hutton, PhD
Materials Science Lead
Scientific Expertise:
- Organic chemistry
- Crystallization processes for commercial applications
- Materials science expert
- API development
- Commercial manufacturing
Focus Area
API (small molecule)
Credentials
Doctor of Philosophy in synthetic organic chemistry from the University of Cambridge, U.K.
Bachelor’s degree in pure & applied chemistry from Strathclyde University in Glasgow, U.K.

Whitepaper
The changing landscape of oncology drug development: Bringing novel lifesaving therapies to patients
Oncology is the fastest-growing, most active sector of drug development. Matching drug products to clinical and commercial needs requires scientific and technological innovation.

Expert
Christy Eatmon
Senior Staff Scientist, R&D
Scientific Expertise:
- Extensive experience in pre-clinical to early phase formulation development of sterile products
- Process engineering expertise to enable technology transfer and scale up of parenteral formulations
- High level knowledge of all phases from drug discovery to commercial manufacturing and commercial launch
- Extensive experience with both small and large molecules, including small molecule formulation for controlled release of poorly water-soluble drugs
Focus Area
Steriles
Credentials
Bachelor’s degree in chemistry from Moravian College in Pennsylvania

Expert

Expert
Paul Jorjorian
Vice President and General Manager, Biologics
Focus Area
Biologics (large molecule)

Expert

Expert

Expert

Expert
Julie Hoffman
Senior Director of Commercial Strategy and Execution
Focus Area
Clinical ancillary management
Clinical trial services

Expert
Janet Williams
Senior Director, Global Supply Chain Management
Focus Area
Clinical ancillary management
Clinical trial services

Expert
Iain McGroarty
Senior Business Development Executive, API Europe
Focus Area
API (small molecule)

Expert
Elena Gontarz, PhD
Manager, Scientific and Technical Affairs
Focus Area
Biologics (large molecule)

Expert
Daniel Baskind
Manager, Scientific and Technical Affairs
Focus Area
Biologics (large molecule)

Expert
Kaspar van den Dries, PhD
Senior Director Science and Innovation, Softgels
Scientific Expertise:
- Formulation & process development
- Solid dose formulation (tablets, capsules, and softgels)
- Late stage process development projects
- Technology transfer
- Regulatory filings
- Solubility enhancement
- Biopharmaceuticals
- Lipid-based formulations
- Quality by design
- Risk assessment and experimental designs
- Process analytical technologies (NIR)
- In vitro – in vivo relationships
Focus Area
Softgels
Credentials
Doctor of Philosophy and Master’s degree in pharmaceutical sciences from the University of Utrecht, NL

Expert
Frank Ritacco, PhD
Director, Scientific and Technical Affairs
Scientific Expertise:
- Highly experienced in the development of cell lines, media, and culture conditions for the production of recombinant proteins and naturalmetabolites, as well as bioreactor operation, scale-up, cGMP manufacturing and technology transfer
- Research experience and skills include both mammalian cell culture and microbial fermentation (bacteria, yeast, filamentous fungi), process development, cellular physiology, and perfusion culture
Focus Area
Biologics (large molecule)
Credentials
Doctor of Philosophy in microbiology and molecular genetics from Rutgers University, NJ
Master of Science in microbiology and molecular genetics from Rutgers University, NJ
Bachelor of Science in biological sciences from Cook College, Rutgers University, NJ

Expert
Peter Poechlauer, PhD
Innovation Manager, Small Molecule API
Scientific Expertise:
- Product development
- Small molecules
- Green chemistry
- Process technology
- Continuous manufacturing & analytics
- Process Intensification
- Organic chemistry
- Biocatalysis
Focus Area
API (small molecule)
Credentials
Doctor of Natural Sciences in organic chemistry & pharmaceutical chemistry from Innsbruck University in Austria

Expert
Jason Mieding
Senior Director, Logistics
Scientific Expertise:
- Highly experienced in supply chain with a particular focus on sales and operations planning, transportation and logistics management, and system enhancements including SAP
- Certified in production and inventory management (American Production and Inventory Control Society)
- Multi-functional leadership experience in manufacturing, quality, and technical areas within pharmaceutical GMP environments
Focus Area
Logistics
Credentials
Bachelor’s degree in production and operations management from Ohio State

Expert
Keirnan LaMarche, PhD
Senior Staff R&D Manufacturing Scientist
Scientific Expertise:
- Tablet compaction
- Powder flow
- Continuous manufacturing of drug products
- Characterized and model drying processes, unit operators
- Fluid bed driers
- One-pot granulators
- Tablet coaters
Focus Area
Oral solid dose
Credentials
Doctor of Philosophy and Master’s degree in chemical engineering from Rutgers University
Bachelor’s degree in engineering, chemical engineering from The Cooper Union for the Advancement of Science & Art

Expert
Sanjay Konagurthu, PhD
Senior Director, Science and Innovation
Scientific Expertise:
- Product development
- Oral drug delivery
- Drug product development
- Pre-formulation, formulation, process development, scale-up, tech transfer, and commercialization
- Solubility enhancement technologies
- Amorphous solid dispersions by spray-drying
- Hot-melt extrusion
- Size-reduction
- Lipid-based systems
- Complexation
- Oral modified release
- Multiparticulate delivery
- Osmotic delivery
- Taste masking
- Fast-dissolving dosage forms
- Lifecycle management
- Designing novel drug delivery systems
- Nano-milling
- Process miniaturization
- Process development and scale-up
- Novel process design & implementation
- Design of experiments
- Technology transfer
Focus Area
Formulation and development
Credentials
Doctor of Philosophy in chemical engineering from the University of Colorado in Boulder
Bachelor’s degree in chemical engineering from the Indian Institute of Technology Madras (Chennai)

Expert
Kevin Kane, PhD
Senior Staff Scientist, R&D
Scientific Expertise:
- Oral drug delivery
- Drug product development
- Pre-formulation, formulation, process development, scale-up, tech transfer, commercialization
- Phase appropriate dosage forms for preclinical and clinical studies
- Solubility enhancement technologies
- Amorphous solid dispersions by spray-drying, hot-melt extrusion and complexation
- Size-reduction and lipid-based systems
- Oral modified release
- Multiparticulate and osmotic delivery
- Matrix tablets
- Lipid modified release
- Injectable drug delivery
- Formulation development for solution and lyophilized vials for small molecules
- IV and depot formulations
- Powder-filled vials
- Technology transfer
Focus Area
Commercial manufacturing
Credentials
Doctor of Philosophy in inorganic chemistry from Ohio University
Post-doctoral Fellow and Visiting Assistant Professor of chemistry at the University of Idaho
Master’s and Bachelor’s degrees in chemistry (polymer and materials) from Texas State University

Expert
Jeff Hou, PhD
Director and Site Head, Scientific & Technical Affairs
Scientific Expertise:
- Biologics development
- Cell line development
- Process development
- Tech transfer
- GMP manufacturing
- Cell biology
- Lead candidate discovery
Focus Area
Biologics (large molecule)
Credentials
Doctor of Philosophy in biotechnology from The University of Queensland
Bachelor’s degree in advanced sciences from the University of New South Wales

Video
Bioprocessing Collaboration Center (BCC)
The new Bioprocessing Collaboration Center (BCC) in St. Louis, Missouri, uniquely positions Thermo Fisher to bring together industry-leading expertise in single-use technologies and biologics product development and manufacturing to deliver unmatched value to our customers.





























